WillD
Expert
- Joined
- Jul 19, 2021
- Messages
- 645
- Reaction score
- 895
- Points
- 93
2-(3-methoxy-5-hydroxyphenyl)-2-methylheptane:
1. To a suspension of 1.4 g (34 mmol, 60% dispersion in oil) of NaH in 14 ml of dry DMF, was added dropwise 4.2 ml (46 mmol) of 1-propanethiol.
2. The reaction was stirred for 30 min at ambient temperature.
3. To this mixture was added 1.32 g (5.28 mmol) of 2-(3,5-dimethoxyphenyl)-2-methylheptane in 7 ml of dry DMF.
4. The resultant was heated at reflux for 5 h.
5. Cooled to ambient temperature and poured into 40 ml of 1M HCl.
6. The solution was extracted with three portions of ether and the ethereal extracts were washed with successive portions of aqueous NaHCO3, brine, dried (MgSO4) and concentrated in vacuo.
7. The crude product was purified to afford 0.96 g (77%) of 2-(3-methoxy-5-hydroxyphenyl)-2-methylheptane as a colorless oil.
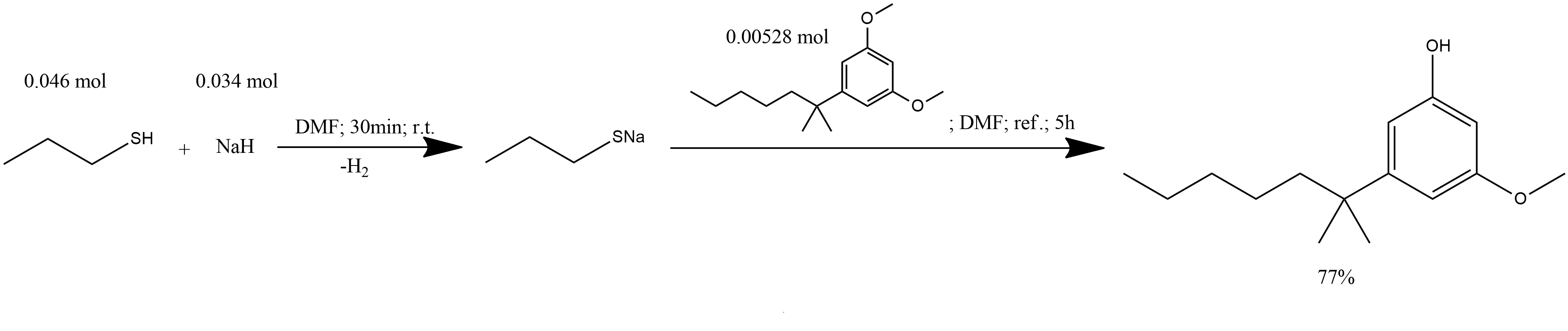
2-(3-methoxyphenyl)-2-methylheptane:
1. To a solution of 1.56 g (6.61 mmol) of 2-(3-hydroxyphenyl-5-methoxyphenyl)-2-methylheptane and 1.08 ml (8.42 mmol) of diethyl phosphite in 4 ml of CCl 4 at 0 *C was added dropwise 1 ml (7.17 mmol) of triethylamine.
2. The solution was stirred at 0 *C for 1 h.
3. Allowed to warm to ambient temperature and stirred for 7 h at ambient temperature.
4. The mixture was diluted with CH2Cl2 and washed with successive solutions of H2O, 1M aqueous NaOH, H2O, 1M HCl, and H2O.
5. The organic layer was dried (MgSO4), concentrated in vacuo and purified to give 2.01 g (93%) of 2-(3-hydroxyphenyl-5-methoxyphenyl)-2-methylheptane diethyl phosphate as a red oil, which was used in the next step without further purification.
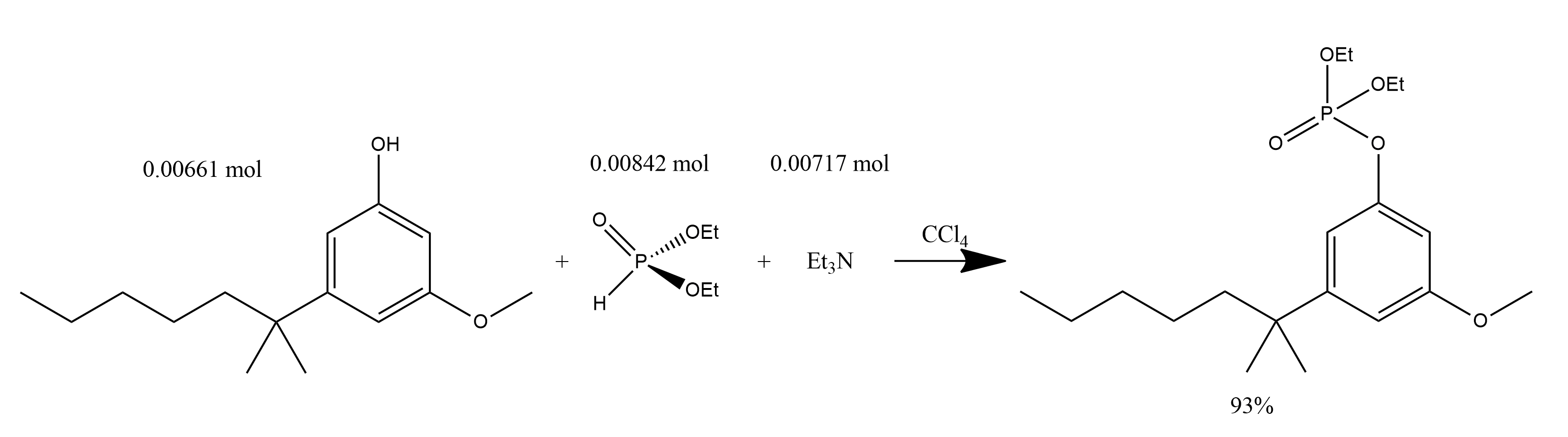
2-(4-Bromo-3-methoxyphenyl)-2-methylheptane:
1. To a solution of 0.281 g (1.27 mmol) of 2-(3-hydroxyphenyl-5-methoxyphenyl)-2-methylheptane diethyl phosphate in 0.5 ml of glacial acetic acid was slowly added 0.065 ml (1.27 mmol) of bromine in 0.5 mL of acetic acid at ambient temperature.
2. The solution was stirred for 2 h at ambient temperature, diluted with 5 ml of water and 3 ml of aqueous NaHCO3.
3. The reaction mixture was extracted with two portions of ether and the ethereal extracts were washed with brine, dried (MgSO4), and concentrated in vacuo.
4. The resultant orange oil was purified to give 0.201 g (79%) of 2-(4-bromo-3-methoxy-phenyl)-2-methylheptane (9) as a colorless oil.
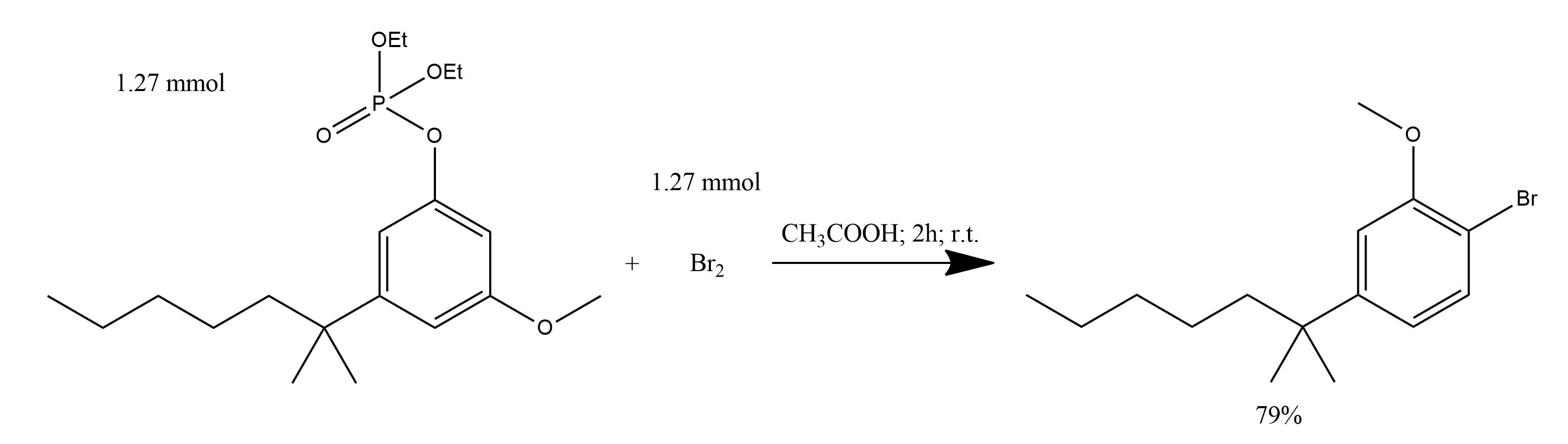
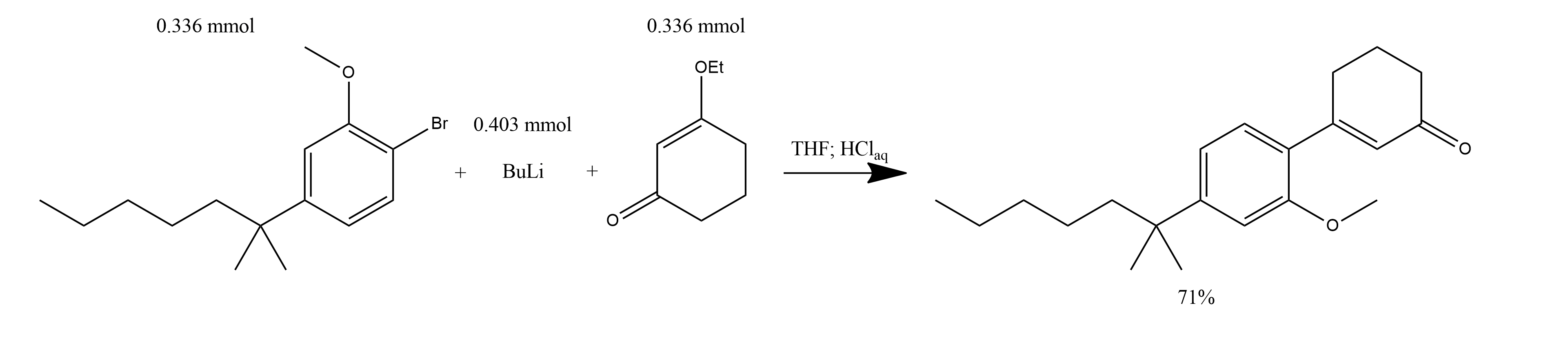
3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohex-2-en-1-one:
1. To a solution of 0.100 g (0.336 mmol) of 2-(4-bromo-3-methoxyphenyl)-2-methylheptane in 3 ml of dry THF at -78*C was added 0.16 ml (0.403 mmol, 2.5M solution in cyclohexane) of n-butyllithium.
2. The mixture was stirred for 30 min.
3. A solution of 0.048 g (0.336 mmol) of 3-ethoxycyclohexen-1-one in 2 ml of dry THF was added dropwise
4. The solution was heated for 4 h at reflux.
5. After cooling to ambient temperature, the reaction was diluted with 15 ml of 10% aqueous HCl.
6. Stirred for 30 min and extracted with two portions of ether.
7. The combined ethereal layers were washed with saturated aqueous NaHCO3, brine, dried (MgSO4).
8. Concentrated in vacuo to give 0.075 g (71%) of 3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohex-2-en-1-one as a yellow oil.
3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohexanone:
1. To 20 ml of liquid ammonia at -78 *C was added 0.004 g (0.579 g atom) of lithium shot.
2. The solution was stirred for 10 min.
3. A solution of 0.072 g (0.23 mmol) of 3-[2-methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohex-2-en-1-one in 25 ml of dry THF was slowly added, and the mixture was stirred at -78 *C for 30 min.
4. The reaction was quenched by the addition of NH4Cl and the ammonia was evaporated at ambient temperature.
5. The mixture was diluted with 10 ml of H2O and extracted with two portions of ether.
6. The ethereal extracts were washed with brine, dried (MgSO4) and concentrated in vacuo.
7. The yellow oil was purified to give 0.056 g (78%) of 3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohexanone as a colorless oil.
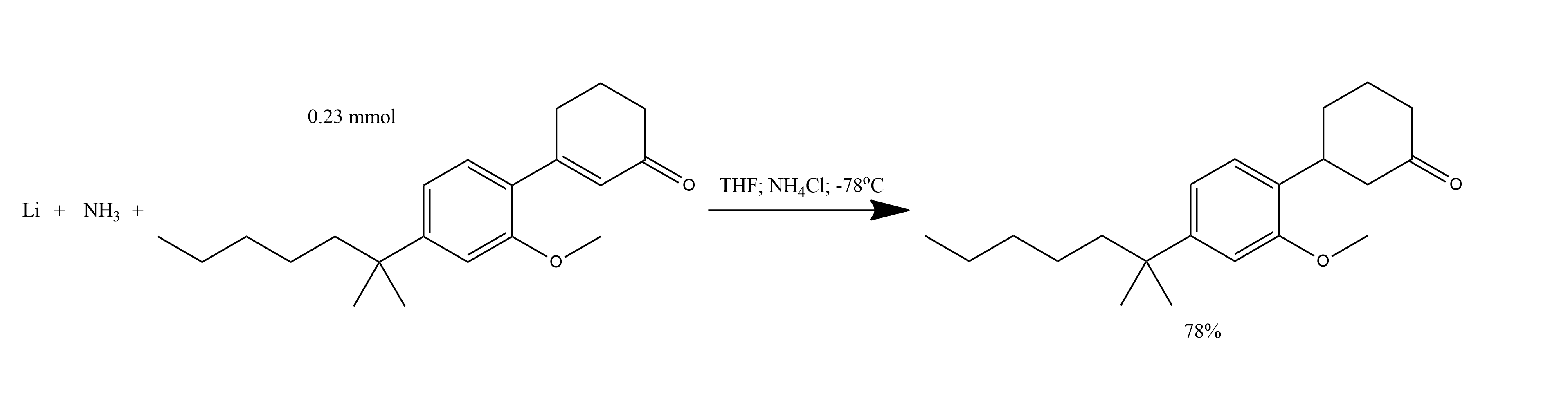
CP-47,497.
Reduction 3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohexanone with NaBH4 and Pd/C-hydrogen.
1. To a suspension of 1.4 g (34 mmol, 60% dispersion in oil) of NaH in 14 ml of dry DMF, was added dropwise 4.2 ml (46 mmol) of 1-propanethiol.
2. The reaction was stirred for 30 min at ambient temperature.
3. To this mixture was added 1.32 g (5.28 mmol) of 2-(3,5-dimethoxyphenyl)-2-methylheptane in 7 ml of dry DMF.
4. The resultant was heated at reflux for 5 h.
5. Cooled to ambient temperature and poured into 40 ml of 1M HCl.
6. The solution was extracted with three portions of ether and the ethereal extracts were washed with successive portions of aqueous NaHCO3, brine, dried (MgSO4) and concentrated in vacuo.
7. The crude product was purified to afford 0.96 g (77%) of 2-(3-methoxy-5-hydroxyphenyl)-2-methylheptane as a colorless oil.
2-(3-methoxyphenyl)-2-methylheptane:
1. To a solution of 1.56 g (6.61 mmol) of 2-(3-hydroxyphenyl-5-methoxyphenyl)-2-methylheptane and 1.08 ml (8.42 mmol) of diethyl phosphite in 4 ml of CCl 4 at 0 *C was added dropwise 1 ml (7.17 mmol) of triethylamine.
2. The solution was stirred at 0 *C for 1 h.
3. Allowed to warm to ambient temperature and stirred for 7 h at ambient temperature.
4. The mixture was diluted with CH2Cl2 and washed with successive solutions of H2O, 1M aqueous NaOH, H2O, 1M HCl, and H2O.
5. The organic layer was dried (MgSO4), concentrated in vacuo and purified to give 2.01 g (93%) of 2-(3-hydroxyphenyl-5-methoxyphenyl)-2-methylheptane diethyl phosphate as a red oil, which was used in the next step without further purification.
2-(4-Bromo-3-methoxyphenyl)-2-methylheptane:
1. To a solution of 0.281 g (1.27 mmol) of 2-(3-hydroxyphenyl-5-methoxyphenyl)-2-methylheptane diethyl phosphate in 0.5 ml of glacial acetic acid was slowly added 0.065 ml (1.27 mmol) of bromine in 0.5 mL of acetic acid at ambient temperature.
2. The solution was stirred for 2 h at ambient temperature, diluted with 5 ml of water and 3 ml of aqueous NaHCO3.
3. The reaction mixture was extracted with two portions of ether and the ethereal extracts were washed with brine, dried (MgSO4), and concentrated in vacuo.
4. The resultant orange oil was purified to give 0.201 g (79%) of 2-(4-bromo-3-methoxy-phenyl)-2-methylheptane (9) as a colorless oil.
3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohex-2-en-1-one:
1. To a solution of 0.100 g (0.336 mmol) of 2-(4-bromo-3-methoxyphenyl)-2-methylheptane in 3 ml of dry THF at -78*C was added 0.16 ml (0.403 mmol, 2.5M solution in cyclohexane) of n-butyllithium.
2. The mixture was stirred for 30 min.
3. A solution of 0.048 g (0.336 mmol) of 3-ethoxycyclohexen-1-one in 2 ml of dry THF was added dropwise
4. The solution was heated for 4 h at reflux.
5. After cooling to ambient temperature, the reaction was diluted with 15 ml of 10% aqueous HCl.
6. Stirred for 30 min and extracted with two portions of ether.
7. The combined ethereal layers were washed with saturated aqueous NaHCO3, brine, dried (MgSO4).
8. Concentrated in vacuo to give 0.075 g (71%) of 3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohex-2-en-1-one as a yellow oil.
3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohexanone:
1. To 20 ml of liquid ammonia at -78 *C was added 0.004 g (0.579 g atom) of lithium shot.
2. The solution was stirred for 10 min.
3. A solution of 0.072 g (0.23 mmol) of 3-[2-methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohex-2-en-1-one in 25 ml of dry THF was slowly added, and the mixture was stirred at -78 *C for 30 min.
4. The reaction was quenched by the addition of NH4Cl and the ammonia was evaporated at ambient temperature.
5. The mixture was diluted with 10 ml of H2O and extracted with two portions of ether.
6. The ethereal extracts were washed with brine, dried (MgSO4) and concentrated in vacuo.
7. The yellow oil was purified to give 0.056 g (78%) of 3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohexanone as a colorless oil.
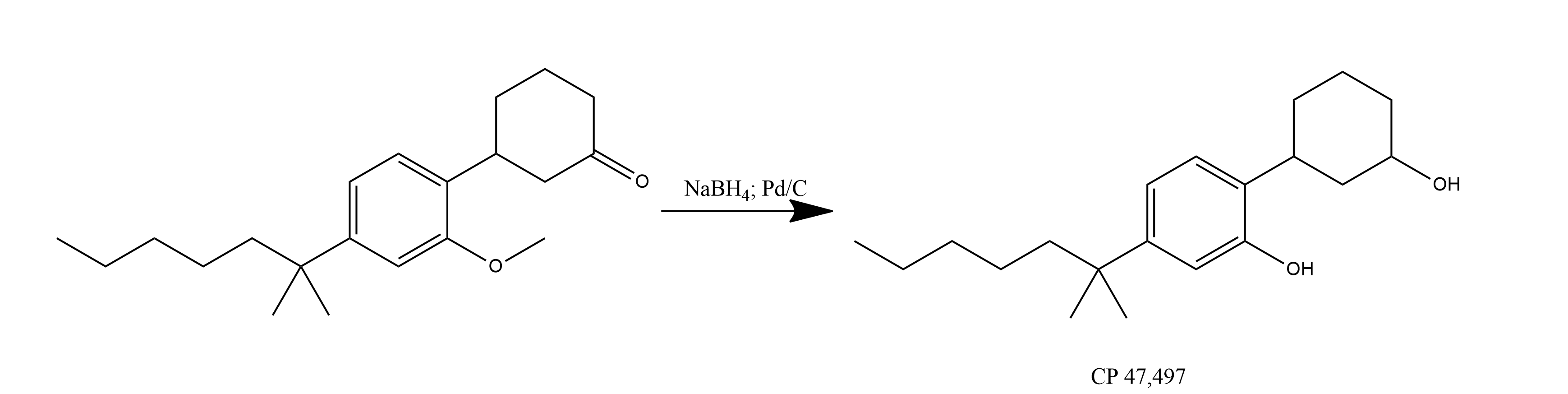
CP-47,497.
Reduction 3-[2-Methoxy-4-(1,1-dimethylhexyl)phenyl]cyclohexanone with NaBH4 and Pd/C-hydrogen.
Last edited by a moderator:
