G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,412
- Solutions
- 3
- Reaction score
- 2,380
- Points
- 113
- Deals
- 1
Introduction.
I want to represent to your attention toluene synthesis method via Freidel-Craft's reaction. This method quite difficult and takes some chemical experience because there are manipulations with aggressive gases, fractional distillation and hazard substances. If you have option to buy toluene (methylbenzene) in chem shop, it would be better than suffer and provide this experiment. In other cases, use this method and be careful, use all safety equipment such as chemical glass, gloves, chemical coat and respiratory mask. Carry on experiments under pull out probe or under exhaust hood in good ventilated premises.
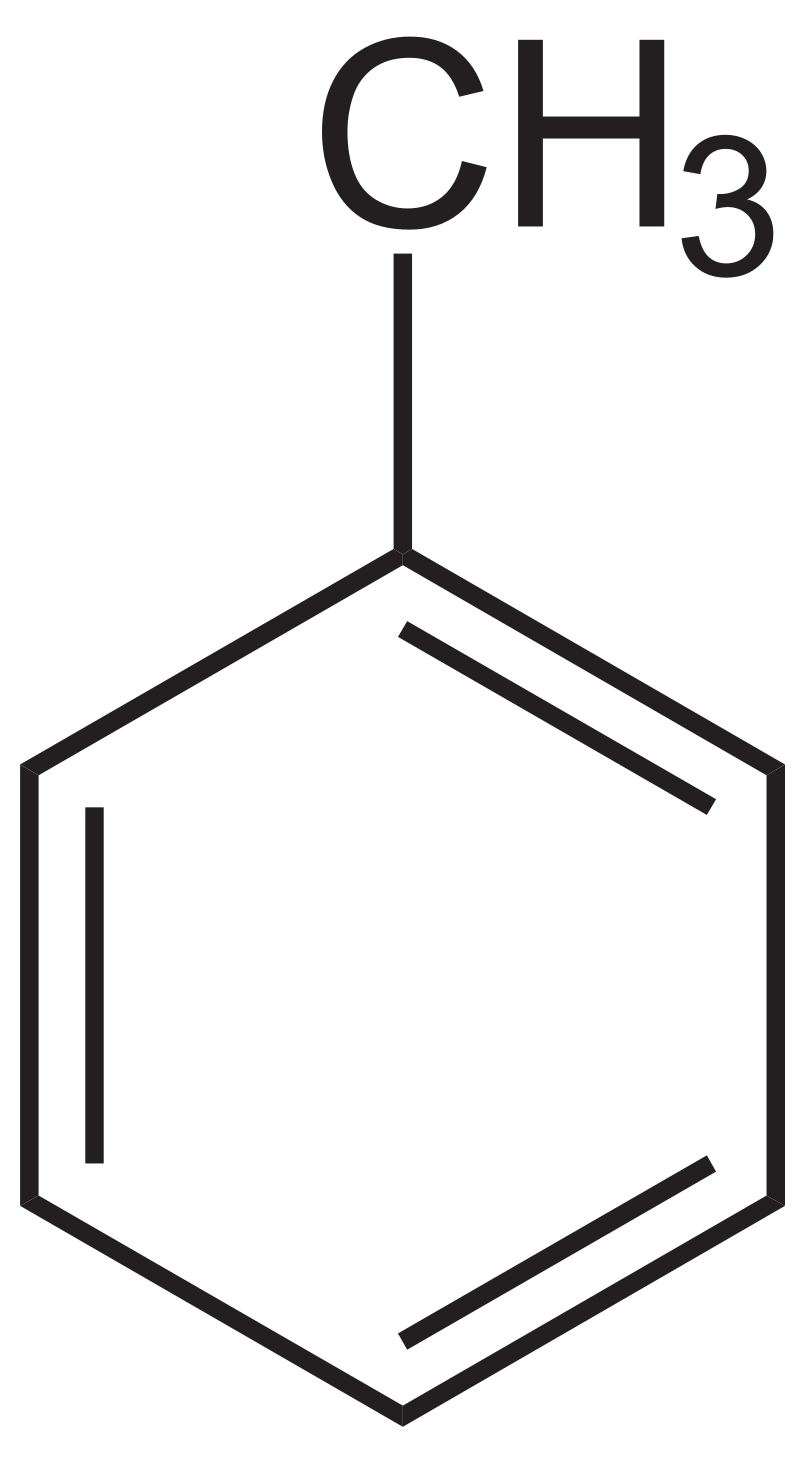
Appearance: colorless liquid with a sweet, pungent, benzene-like odor;Boiling Point: 110.6 °C/760 mm Hg;
Melting Point: -95 °C;
Molecular Weight: 92.141 g/mol;
Density: 0.86694 g/ml (20 °C).
Equipment and glassware:
- 2 L x3 Two-neck round bottom flask;
- 0.5 L Drip funnel;
- x2 Heating mantle;
- x2 Calcium chloride tube;
- Glass tubes;
- Glass tube connector to flask throats;
- Flush flask;
- x2 Reflux condenser;
- Water-jet aspirator;
- Distillation apparatus with vigreux column;
- Retort stand and clamp for securing apparatus;
- Laboratory scale (1 — 200 g is suitable);
- 100 ml x2 and 200 ml x2 Erlenmeyer flasks with cap;
- 1 L Measuring cylinder;
- 200 ml x2; 100 ml x2 Beakers;
- Conventional funnel.
Reagents:
- 300 ml Sulfuric acid conc. (H2SO4);
- 200 g Sodium chloride (NaCl);
- 500 ml Methanol (MeOH);
- 30 g Anhydrous zinc chloride (ZnCl2);
- 92 g Anhydrous aluminum chloride (AlCl3);
- 500 ml Benzene.
Procedure:
1. Firstly, we are assembling the installation for toluene synthesis. First step is assembling the device for hydrochloric acid gas synthesis. A two-neck round bottom flask connected with a drip funnel with sulfuric acid (H2SO4) (control the amount of gaseous hydrogen chloride). Place sodium chloride (NaCl) on the bottom of the flask and place this flask on a heating mantle. We connect the outlet from the flask through a calcium chloride tube and through a glass tube we introduce it to the bottom of the second two-necked round bottom flask.3. Connect the outlet from the wash bottle to a calcium chloride tube, and then connect it to a glass tube touching the bottom of the third two-necked flask.
4. Pour 500 ml of benzene into the third flask, add 92 g of anhydrous aluminum chloride (AlCl3) and close the throat with a reflux condenser, the outlet of which is connected to a water-jet pump.
5. We turn on the apparatus by turning on the heating of benzene and methanol. As soon as the methanol starts to boil, open the drip funnel with sulfuric acid and start generating hydrogen chloride gas. Hydrogen chloride dried through calcium chloride tube, getting into boiling methanol in the presence of ZnCl2 catalyst forms monochloromethane gas (CH3Cl). Dried monochloromethane gas enters the flask with benzene and forms toluene by the Friedel-Crafts alkylation mechanism in the presence of AlCl3 catalyst.
4. Pour 500 ml of benzene into the third flask, add 92 g of anhydrous aluminum chloride (AlCl3) and close the throat with a reflux condenser, the outlet of which is connected to a water-jet pump.
5. We turn on the apparatus by turning on the heating of benzene and methanol. As soon as the methanol starts to boil, open the drip funnel with sulfuric acid and start generating hydrogen chloride gas. Hydrogen chloride dried through calcium chloride tube, getting into boiling methanol in the presence of ZnCl2 catalyst forms monochloromethane gas (CH3Cl). Dried monochloromethane gas enters the flask with benzene and forms toluene by the Friedel-Crafts alkylation mechanism in the presence of AlCl3 catalyst.
Boiling points of pure substances:
Benzene Bp = 80.1 °C;Toluene Bp = 111 °C;
Ortho-xylene Bp = 144 °C;
Para-xylene Bp = 138 °C;
Meta-xylene Bp = 139 °C.
Last edited:
