Brain
Expert Pharmacologist
- Joined
- Jul 6, 2021
- Messages
- 223
- Reaction score
- 197
- Points
- 43
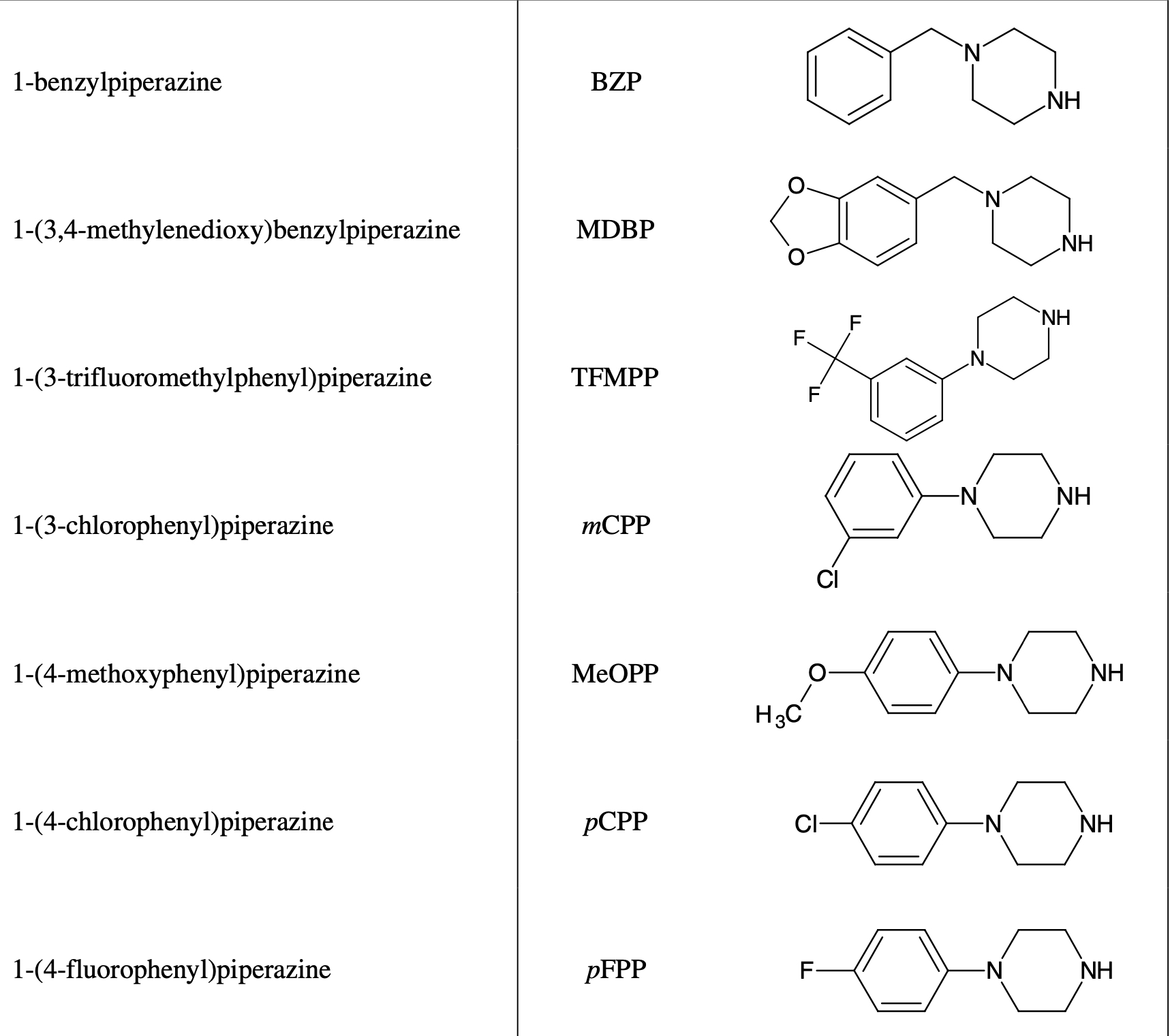
Benzylpiperazine – is a recreational psychoactive substance of piperazine group, which has psychostimulative and euphorogenic effects, similar to that of amphetamine. Piperazine derivatives make up a group of chemically modified designer drugs, derived from piperazine, which is a six-membered ring with two oppositely arranged nitrogen atoms. Benzylpiperazines include N-benzylpiperazine (BZP) and 1-(3,4-methylenedioxybenzyl)-piperazine (MDBP). Common phenylpiperazines are 1-(3-trifluoromethylphenyl) piperazine (TFMPP), 1-(3-chlorophenyl) piperazine. Chemical modification of piperazine compounds allows illegal manufacturers to workaround government laws and promotes their wide distribution under various names: “Rapture”, “Frenzy”, “Bliss”, “Charge”, “Herbal ecstasy”, “A2”, “Legal X”, and “Legal E”. Initially, BZP was synthesized in 1944 by the company Burroughs, Wellcome & Co from Wellcome Research Laboratories in Great Britain. It was under trials as an anthelmintic agent for the treatment of intestinal infestations of roundworms, but due to its greater effectiveness and fewer side effects, preference was given to piperazine. The benzylpiperazine group includes: 2C-B-BZP, 3-Me-BZP, Befuraline, Bifeprunox, Buclizine, Chlorbenzoxamine, DBZP, Fipexide, Imatinib, MBZP, MDBZP, Meclozine, MeOP, Piberaline, Piribedil, RN-1747, Sunifiram, Trimetazidine, TFMCPP, Vesnarinone.

In the 1970s BZP was considered a potential antidepressant, but it wasn't chosen as such due to a great potential for abuse. In late 1990s New Zealand youth popularized it as a legal party drug with stimulating effects (confidence, talkativeness, euphoria, cheerfulness, boost in energy and socialization), that is why it has become so widespread. In the 1980s benzylpiperazine derivative - N-benzyl-piperazine-picolinyl fumarate was synthesized as an antidepressant by scientists from Semmelweis University of Medicine in Hungary. It was called EGYT-475. Due to its psychoactive properties, legal status in many countries and deceptive safety, recreational use of piperazine derivatives gained popularity as an alternative to amphetamine, despite a lot of experimental, clinical and epidemiological studies, in which it was associated with severe serotonin syndrome, hepatotoxicity, mental disorders and high potential of abuse.
New Zealand users considered legal status a guarantee of BZP purity, while manufacturers synthesized it with no control. Product labels made a false impression on the buyer that they knew for sure what they were getting. Many users underestimated the effects of the pills and described them as moderate. Moreover, pills containing BZP were socially acceptable and widely available because there were no law restrictions. Eventually, the idea was put forward that BZP encouraged the user to use other illegal drugs (“gateway”) or provided the illegal drug users a legal alternative. It is mentioned in some studies, that not long after befuraline - (DIV-145; 1-benzofuran-2-yl-(4-benzylpiperazin-1-yl)-methanone) was synthesized and put under clinical trial as an antidepressant. Benzylpiperazine is used orally in form of capsules, tablets, liquid, intranasally – in form of powder.
BZP is a diamine, which doesn't have stereoisomers. The substance is manufactured in the form of free base or hydrochloride salt, has a molecular formula C11H16N2 and molecular weight of 249.19 g/mol. The main form has the appearance of yellowish-green liquid, which has a constant (pKA) of 9.02 (20 °C). Hydrochloride salt has the appearance of white solid substance, soluble in water, which irritates eyes, respiratory system and skin. It is easily synthesized as a result of the reaction between piperazine monohydrochloride and benzyl chloride, which are easily available chemical compounds.
Pharmacokinetics and pharmacodynamics.
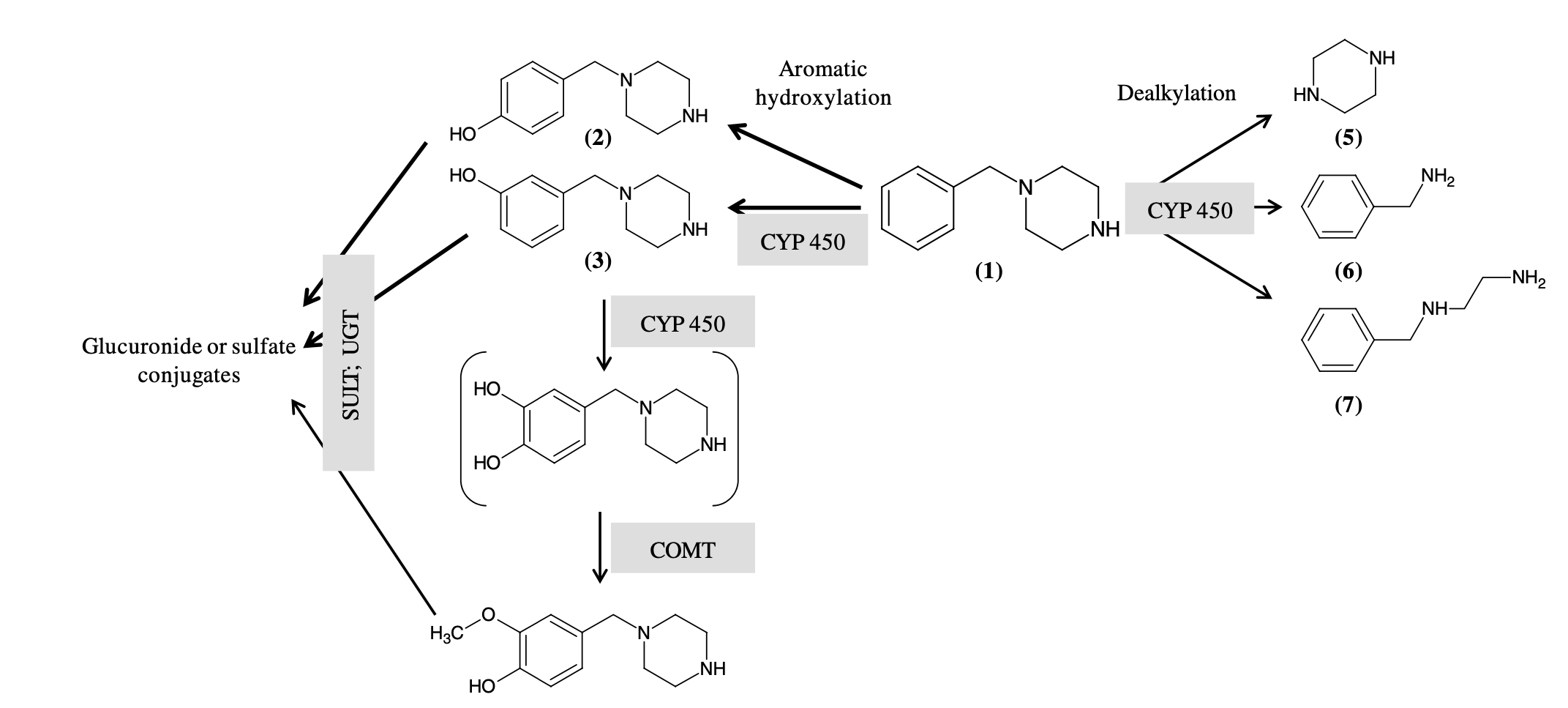
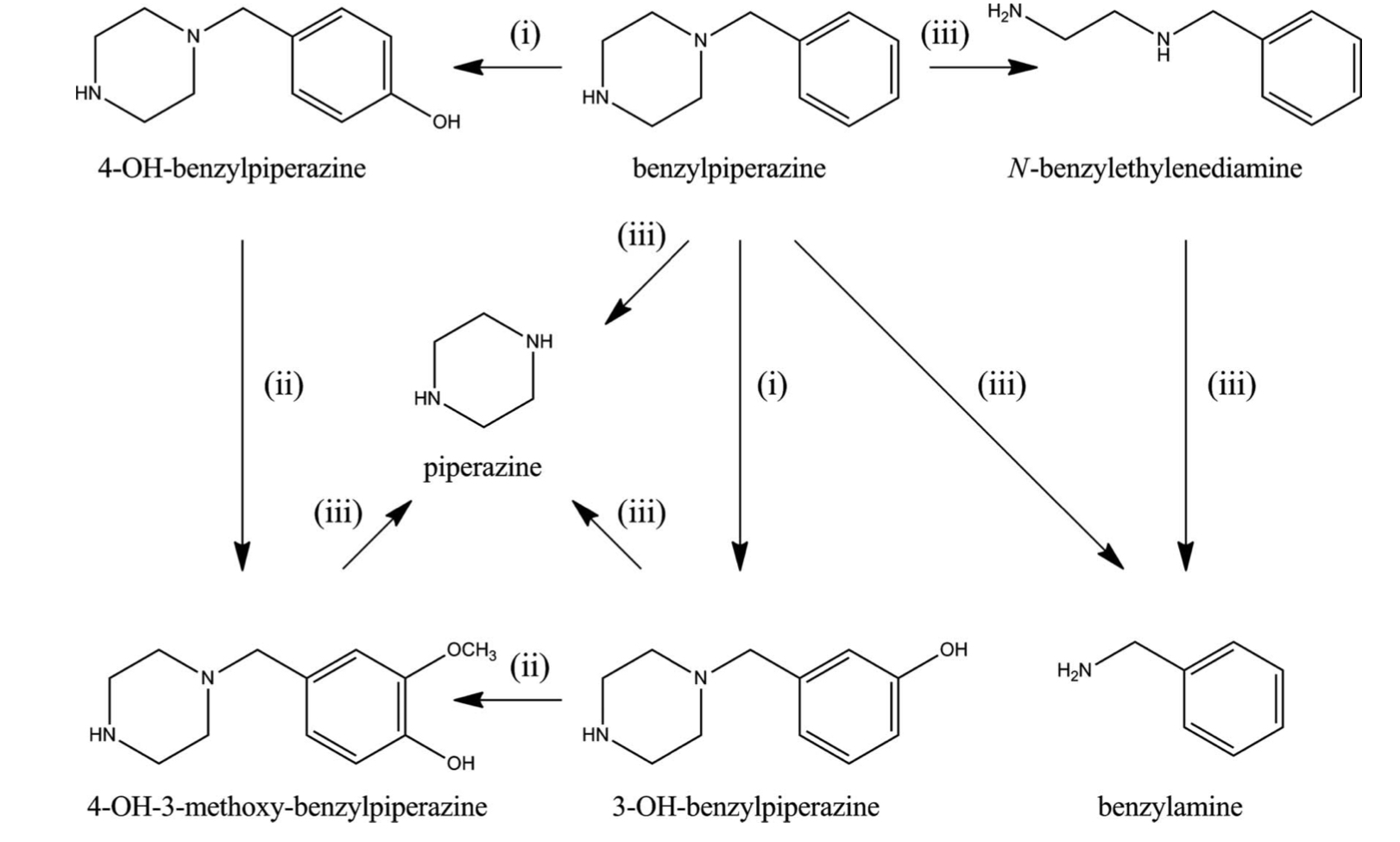
Pharmacokinetic parameters of BZP are not studied fully, but it is a fact that it undergoes hydroxylation and dealkylation in the first phase and has three metabolic targets: aromatic ring, benzyl carbon and piperazine compound. Aromatic ring is transformed through the process of single aromatic hydroxylation, as a result 4-hydroxy-BZP, 3-hydroxy-BZP are formed, and also through the process of double aromatic hydroxylation with subsequent methylation and formation of 4-hydroxy-3-methoxy-BZP. Also, metabolic dealkylation of benzyl and piperazine carbons leads to the formation of benzylamine and N-benzylethylenediamine. Hydroxylated compounds can also be involved in the reactions of the second phase of metabolism, partially transformed and excreted in the form of sulfate or glucoronide compounds. According to the recent studies, a number of enzymes are involved in BZP metabolism: P450 (CYP), which catalyzes reactions of the first phase (hydroxylation and dealkylation), catechol-O-methyl-transferase, which catalyzes methylation of the dihydroxy metabolite, sulfotransferase (SULT) and uridine-diphosphate-glucuronyl-transferase, responsible for the formation of sulfate and glucoronide conjugates. In addition, the occurrence of genetic enzymatic polymorphisms may contribute to the interindividual variability of benzylpiperazine toxicity level. As for the role of CYP enzymes, the following position of activity of its isoforms was revealed in vitro studies: CYP2D6 (28 %)<CYP3A4 (54%)< CYP1A2 (74%).
In studies of Tsutsumi 2006 it was stated that during 48 hours after administration of BZP at a dose of 6 mg/kg BZP, 25% of the substance was excreted unchanged. Almost 50% of the metabolite was excreted in the form of glucoronide conjugates and less in the form of sulfate conjugates. Peak concentration of BZP in urine was determined after 4 hours since administration. It could not be detected at all after 48 hours, at the same time peak concentration of metabolites was determined after 8 hours. Extremely high toxicity of metabolites 3-OH-BZP and 4-OH-BZP was proved for liver and kidneys. Maximum concentration in plasma was reached after 75 minutes, indicator AUC 212,000 ng/ml with a limit of quantification of 5 ng/ml and a maximum concentration in plasma was 262.7 ng/ml. Half-life elimination period was 5.5 hours, clearance was 99 l/hour. About 6% of the dose (6 mg) was excreted in an unconjugated form, and unconjugated metabolites 4-OH-BZP and 3-OH- BZP made up only 0.11%.In the 1970s BZP was considered a potential antidepressant, but it wasn't chosen as such due to a great potential for abuse. In late 1990s New Zealand youth popularized it as a legal party drug with stimulating effects (confidence, talkativeness, euphoria, cheerfulness, boost in energy and socialization), that is why it has become so widespread. In the 1980s benzylpiperazine derivative - N-benzyl-piperazine-picolinyl fumarate was synthesized as an antidepressant by scientists from Semmelweis University of Medicine in Hungary. It was called EGYT-475. Due to its psychoactive properties, legal status in many countries and deceptive safety, recreational use of piperazine derivatives gained popularity as an alternative to amphetamine, despite a lot of experimental, clinical and epidemiological studies, in which it was associated with severe serotonin syndrome, hepatotoxicity, mental disorders and high potential of abuse.
New Zealand users considered legal status a guarantee of BZP purity, while manufacturers synthesized it with no control. Product labels made a false impression on the buyer that they knew for sure what they were getting. Many users underestimated the effects of the pills and described them as moderate. Moreover, pills containing BZP were socially acceptable and widely available because there were no law restrictions. Eventually, the idea was put forward that BZP encouraged the user to use other illegal drugs (“gateway”) or provided the illegal drug users a legal alternative. It is mentioned in some studies, that not long after befuraline - (DIV-145; 1-benzofuran-2-yl-(4-benzylpiperazin-1-yl)-methanone) was synthesized and put under clinical trial as an antidepressant. Benzylpiperazine is used orally in form of capsules, tablets, liquid, intranasally – in form of powder.
BZP is a diamine, which doesn't have stereoisomers. The substance is manufactured in the form of free base or hydrochloride salt, has a molecular formula C11H16N2 and molecular weight of 249.19 g/mol. The main form has the appearance of yellowish-green liquid, which has a constant (pKA) of 9.02 (20 °C). Hydrochloride salt has the appearance of white solid substance, soluble in water, which irritates eyes, respiratory system and skin. It is easily synthesized as a result of the reaction between piperazine monohydrochloride and benzyl chloride, which are easily available chemical compounds.
Pharmacokinetics and pharmacodynamics.
Pharmacokinetic parameters of BZP are not studied fully, but it is a fact that it undergoes hydroxylation and dealkylation in the first phase and has three metabolic targets: aromatic ring, benzyl carbon and piperazine compound. Aromatic ring is transformed through the process of single aromatic hydroxylation, as a result 4-hydroxy-BZP, 3-hydroxy-BZP are formed, and also through the process of double aromatic hydroxylation with subsequent methylation and formation of 4-hydroxy-3-methoxy-BZP. Also, metabolic dealkylation of benzyl and piperazine carbons leads to the formation of benzylamine and N-benzylethylenediamine. Hydroxylated compounds can also be involved in the reactions of the second phase of metabolism, partially transformed and excreted in the form of sulfate or glucoronide compounds. According to the recent studies, a number of enzymes are involved in BZP metabolism: P450 (CYP), which catalyzes reactions of the first phase (hydroxylation and dealkylation), catechol-O-methyl-transferase, which catalyzes methylation of the dihydroxy metabolite, sulfotransferase (SULT) and uridine-diphosphate-glucuronyl-transferase, responsible for the formation of sulfate and glucoronide conjugates. In addition, the occurrence of genetic enzymatic polymorphisms may contribute to the interindividual variability of benzylpiperazine toxicity level. As for the role of CYP enzymes, the following position of activity of its isoforms was revealed in vitro studies: CYP2D6 (28 %)<CYP3A4 (54%)< CYP1A2 (74%).
The fact of inhibition of BZP metabolism by 60% has been proven by means of TFMPP administrated together wth BZP. In 1986, it was revealed that BZP metabolites were able to potentiate 3H-noradrenaline (3H-NA) release, they were also revealed to have some antagonist action in regard to 5-HT. According to the study results of Nagai in 2007, BZP induces DAT-dependant release of [3H]MPP+, but doesn't influence release of [3H]5-HT caused by SERT (as opposed to MDMA and TFMPP). In dose-dependent form, BZP inhibits NA reuptake and increases levels of DA and 5-HT, but 3 times less than MDMA does. In studies on human embryonic kidney cells, it was shown that BZP inhibits serotonin transporters, preventing reuptake of monoamines (DA, NE and 5-HT to a lesser extent). Weak hallucinogenic effect after use at a high dose is caused by BZP binding to 5-HT2A receptor. 5-HT2B is localized in the gastrointestinal tract, so it is responsible for the peripheral side effects such as epigastric pain, nausea and vomiting. Moreover, BZP binding to 5-HT3 receptor causes migraine headaches.
P 25% of the substance was excreted unchanged. Almost 50% of the metabolite was excreted in the form of glucoronide conjugates and less in the form of sulfate conjugates. Peak concentration of BZP in urine was determined after 4 hours since administration. It could not be detected at all after 48 hours, at the same time peak concentration of metabolites was determined after 8 hours. Extremely high toxicity of metabolites 3-OH-BZP and 4-OH-BZP was proved for liver and kidneys. Maximum concentration in plasma was reached after 75 minutes, indicator AUC 212,000 ng/ml with a limit of quantification of 5 ng/ml and a maximum concentration in plasma was 262.7 ng/ml. Half-life elimination period was 5.5 hours, clearance was 99 l/hour. About 6% of the dose (6 mg) was excreted in an unconjugated form, and unconjugated metabolites 4-OH-BZP and 3-OH- BZP made up only 0.11%.
The fact of inhibition of BZP metabolism by 60% has been proven by means of TFMPP administrated together wth BZP. In 1986, it was revealed that BZP metabolites were able to potentiate 3H-noradrenaline (3H-NA) release, they were also revealed to have some antagonist action in regard to 5-HT. According to the study results of Nagai in 2007, BZP induces DAT-dependant release of [3H]MPP+, but doesn't influence release of [3H]5-HT caused by SERT (as opposed to MDMA and TFMPP). In dose-dependent form, BZP inhibits NA reuptake and increases levels of DA and 5-HT, but 3 times less than MDMA does. In studies on human embryonic kidney cells, it was shown that BZP inhibits serotonin transporters, preventing reuptake of monoamines (DA, NE and 5-HT to a lesser extent). Weak hallucinogenic effect after use at a high dose is caused by BZP binding to 5-HT2A receptor. 5-HT2B is localized in the gastrointestinal tract, so it is responsible for the peripheral side effects such as epigastric pain, nausea and vomiting. Moreover, BZP binding to 5-HT3 receptor causes migraine headaches.
Clinical effects of benzylpiperazine.
Desirable positive effects of benzylpiperazine include the very same effects, which are associated with amphetamine use or with use of MDMA at low doses: empathogenicity, minor euphoria, happiness, increased efficiency, good mood, psychostimulation, decreased appetite, "spontaneous bodily sensations", "cognitive euphoria" and "analysis enhancement", "thought organization" and "thought acceleration", increased libido, as well as "brightness alteration" and transformations, when visually for a fraction of a second or for several seconds in the area of the minimum viewing angle metamorphoses of people's faces or objects can be visualized that are transformed into the normal when focusing.
As for the study of acute toxicity, clinical presentation is characterized by severity and frequency of the symptoms listed below in descending order: palpitation, tachycardia, arterial hypertension, psychomotor agitation, confusion, anxiety and anxiety, headache, tremor, mydriasis, urinary retention, gastrointestinal disorders, including nausea and vomiting, abdominal pain or discomfort. More severe symptoms, which are predictors of the acute toxicity: hyperthermia, myoclonic twitching, extrapyramidal manifestations, hyperventilation, respiratory failure, convulsions. In case of a high dose and a history of disease, there is a risk of serotonin syndrome development, which manifests after severe motor activity and hyperthermia and can lead to rhabdomyolysis, renal failure with the development of metabolic acidosis, hypoglycemia, liver failure and DIC syndrome.
Other undesirable negative effects include the following clinical symptoms: increased heart rate and blood pressure, dehydration, dry mouth, nausea and vomiting, reflex syncope, spasm of peripheral small vessels, transient erectile dysfunction, anxiety and paranoia, cognitive fatigue, irritability, "restless legs", sleep disturbance with an increase in the duration of the REM sleep phase, suppression of motivation, illusions and hallucinations, impaired consciousness, blurred vision, dysphoria, trism and bruxism, shortness of breath, paresthesia, itching and sweating, muscle and joint pain; during a laboratory tests, hyponatremia is detected in the blood, an increase in the concentration of antidiuretic hormone, ECG shows sinus tachycardia, atrioventricular conduction disorder, prolongation of the QT interval.
Method of use and doses.
Recreational dose of benzylpiperazine for intranasal administration starts at 0.5 mg/kg, which is associated with minimal effects, including psychostimulation, weak euphorogenic effect and minimal side effects such as increased sweating, agitation, bruxism, mydriasis. Medium doses of benzylpiperazine vary from 1.5 to 3.25 mg/kg. Effects manifest after 10 minutes and reach peak in 1 hour after use. They gradually and slowly fade into post-effects period. When medium doses are used, depending on purity of the substance and frequency of use, stable pronounced effects occur, which are accompanied by certain side effects listed above. The manifestation probability of at least 6 side effects is almost 100%. Intramuscular and intravenous administration of benzylpiperazine is prohibited due to a high local toxicity of the substance. When administered orally, it is recommended to use gelatin capsules, by single intake at a starting dose no more than 2.5 mg/kg. The time of onset of initial effects varies from 25 to 45 minutes, and the peak is reached after 1.7-2 hours.
Special instructions.
In case of severe arousal and agitation, and paranoia caused by benzylpiperazine it is recommended to use first-line therapy, which includes benzodiazepines administered intramuscularly; second-line therapy are antipsychotics, and the first choice drug is droperidol, since it has fewer side effects (absence of QT prolongation and extrapyramidal disorders). Doses of tranquilizers should start at 5-10 mg, and if necessary, after 30 minutes, the injection can be repeated with a dose reduction up to 50%. In case of tachycardia up to 120 beats per minute, or an increase in blood pressure up to 160/90 mmHg, correction with benzodiazepines is also recommended, and second-line drugs, in this case, include: isosorbide dinitrate, nitroglycerin, or clonidine. It is categorically not recommended to use b-blockers as treatment due to the fact that they can paradoxically increase blood pressure and worsen the general somatic status of a patient with benzylpiperazine overdose (and other piperazines as well). Increased motor activity can lead to hyperthermia and subsequent depletion of fluid and electrolyte reserves. Therefore, it is necessary to carefully monitor the fluid balance and rehydrate with chloride-bicarbonate-sodium liquid, which is available in any food store. In case of hyperthermia more than 38.5 degrees Celsius it is necessary to call an ambulance because it is a predictor of a severe serotonin syndrome, especially if hyperthermia lasts more than 20 minutes and appeared spontaneously, without any physical activity. In case of a stable hyperthermia not exceeding 38 degrees Celsius, treatment with benzodiazepines at low doses and rest are usually enough.
Complications associated with benzylpiperazine use.
The most common symptoms of overdose are the following:The fact of inhibition of BZP metabolism by 60% has been proven by means of TFMPP administrated together wth BZP. In 1986, it was revealed that BZP metabolites were able to potentiate 3H-noradrenaline (3H-NA) release, they were also revealed to have some antagonist action in regard to 5-HT. According to the study results of Nagai in 2007, BZP induces DAT-dependant release of [3H]MPP+, but doesn't influence release of [3H]5-HT caused by SERT (as opposed to MDMA and TFMPP). In dose-dependent form, BZP inhibits NA reuptake and increases levels of DA and 5-HT, but 3 times less than MDMA does. In studies on human embryonic kidney cells, it was shown that BZP inhibits serotonin transporters, preventing reuptake of monoamines (DA, NE and 5-HT to a lesser extent). Weak hallucinogenic effect after use at a high dose is caused by BZP binding to 5-HT2A receptor. 5-HT2B is localized in the gastrointestinal tract, so it is responsible for the peripheral side effects such as epigastric pain, nausea and vomiting. Moreover, BZP binding to 5-HT3 receptor causes migraine headaches.
Clinical effects of benzylpiperazine.
Desirable positive effects of benzylpiperazine include the very same effects, which are associated with amphetamine use or with use of MDMA at low doses: empathogenicity, minor euphoria, happiness, increased efficiency, good mood, psychostimulation, decreased appetite, "spontaneous bodily sensations", "cognitive euphoria" and "analysis enhancement", "thought organization" and "thought acceleration", increased libido, as well as "brightness alteration" and transformations, when visually for a fraction of a second or for several seconds in the area of the minimum viewing angle metamorphoses of people's faces or objects can be visualized that are transformed into the normal when focusing.
As for the study of acute toxicity, clinical presentation is characterized by severity and frequency of the symptoms listed below in descending order: palpitation, tachycardia, arterial hypertension, psychomotor agitation, confusion, anxiety and anxiety, headache, tremor, mydriasis, urinary retention, gastrointestinal disorders, including nausea and vomiting, abdominal pain or discomfort. More severe symptoms, which are predictors of the acute toxicity: hyperthermia, myoclonic twitching, extrapyramidal manifestations, hyperventilation, respiratory failure, convulsions. In case of a high dose and a history of disease, there is a risk of serotonin syndrome development, which manifests after severe motor activity and hyperthermia and can lead to rhabdomyolysis, renal failure with the development of metabolic acidosis, hypoglycemia, liver failure and DIC syndrome.
Other undesirable negative effects include the following clinical symptoms: increased heart rate and blood pressure, dehydration, dry mouth, nausea and vomiting, reflex syncope, spasm of peripheral small vessels, transient erectile dysfunction, anxiety and paranoia, cognitive fatigue, irritability, "restless legs", sleep disturbance with an increase in the duration of the REM sleep phase, suppression of motivation, illusions and hallucinations, impaired consciousness, blurred vision, dysphoria, trism and bruxism, shortness of breath, paresthesia, itching and sweating, muscle and joint pain; during a laboratory tests, hyponatremia is detected in the blood, an increase in the concentration of antidiuretic hormone, ECG shows sinus tachycardia, atrioventricular conduction disorder, prolongation of the QT interval.
Method of use and doses.
Recreational dose of benzylpiperazine for intranasal administration starts at 0.5 mg/kg, which is associated with minimal effects, including psychostimulation, weak euphorogenic effect and minimal side effects such as increased sweating, agitation, bruxism, mydriasis. Medium doses of benzylpiperazine vary from 1.5 to 3.25 mg/kg. Effects manifest after 10 minutes and reach peak in 1 hour after use. They gradually and slowly fade into post-effects period. When medium doses are used, depending on purity of the substance and frequency of use, stable pronounced effects occur, which are accompanied by certain side effects listed above. The manifestation probability of at least 6 side effects is almost 100%. Intramuscular and intravenous administration of benzylpiperazine is prohibited due to a high local toxicity of the substance. When administered orally, it is recommended to use gelatin capsules, by single intake at a starting dose no more than 2.5 mg/kg. The time of onset of initial effects varies from 25 to 45 minutes, and the peak is reached after 1.7-2 hours.
Special instructions.
In case of severe arousal and agitation, and paranoia caused by benzylpiperazine it is recommended to use first-line therapy, which includes benzodiazepines administered intramuscularly; second-line therapy are antipsychotics, and the first choice drug is droperidol, since it has fewer side effects (absence of QT prolongation and extrapyramidal disorders). Doses of tranquilizers should start at 5-10 mg, and if necessary, after 30 minutes, the injection can be repeated with a dose reduction up to 50%. In case of tachycardia up to 120 beats per minute, or an increase in blood pressure up to 160/90 mmHg, correction with benzodiazepines is also recommended, and second-line drugs, in this case, include: isosorbide dinitrate, nitroglycerin, or clonidine. It is categorically not recommended to use b-blockers as treatment due to the fact that they can paradoxically increase blood pressure and worsen the general somatic status of a patient with benzylpiperazine overdose (and other piperazines as well). Increased motor activity can lead to hyperthermia and subsequent depletion of fluid and electrolyte reserves. Therefore, it is necessary to carefully monitor the fluid balance and rehydrate with chloride-bicarbonate-sodium liquid, which is available in any food store. In case of hyperthermia more than 38.5 degrees Celsius it is necessary to call an ambulance because it is a predictor of a severe serotonin syndrome, especially if hyperthermia lasts more than 20 minutes and appeared spontaneously, without any physical activity. In case of a stable hyperthermia not exceeding 38 degrees Celsius, treatment with benzodiazepines at low doses and rest are usually enough.
Complications associated with benzylpiperazine use.
1. Intense headache (localized or non-localized, often pulsating) that occurs 10-30 minutes after use and lasts more than half an hour, often accompanied by nausea and vomiting.
2. Sternum pain, discomfort in the left hypochondrium, chest area on the left, irradiation of pain to the left, in the left upper limb, left clavicle, decreased superficial sensation in the left parts.
3. Panic attacks, psychosis, anxiety, depersonalization/derealization.
4. An increase in the pulse rate of more than 110 per minute, an increase in blood pressure of more than 140/95 mmHg.
5. An increase in body temperature of more than 37.5 C and hyperthermia lasting more than one hour after use.
6. Fine tremor, convulsions, impaired consciousness up to a coma.
7. Acute coronary syndrome.
8. Sudden cardiac death.
9. Serotonin syndrome.
First aid for overdose.
Indications for going to the hospital or calling an ambulance: impaired or absent consciousness, impaired speech, motor activity, lack of orientation in space and time, severe pain behind the sternum lasting more than half an hour, an increase in body temperature of more than 38.0 C or hyperthermia lasting more than half an hour, an increase in blood pressure of more than 180/110 mmHg with no effect of hypotensive therapy.
1. The treatment of patients with blood pressure of more than 140/95 mm Hg includes one tablet of a benzodiazepine or a beta-blocker without intrinsic sympathomimetic activity, one tablet of an ACE inhibitor, after 30 minutes - one tablet of a tranquilizer (0.25 mg of alprazolam).
2. In patients with intense anxiety, panic attack, psychosis: one tablet of a tranquilizer and one tablet of a neuroleptic with a sedative effect, psychological help, emergency psychotherapy.
3. In patients with sternum pain, discomfort in the chest: one tablet of slow calcium channel blockers of the third generation, reflexively reducing the heart rate, one tablet of an ACE inhibitor of the 3rd generation OR one tablet of an agonist of imidazoline effects OR one tablet ; if the pain syndrome does not become less intense within 20 minutes, then it is recommended to go to the hospital.
4. When there is an increase in body temperature of no more than 37.5 C, dynamic observation for half an hour is ordered. Pharmacological treatment is not required. If hyperthermia persists for more than half of an hour (in the absence of external causes), then it is recommended to go to the hospital.
5. In patients with intense headaches, it is recommended to use antispasmodics in combination with sedative herbal remedies or tranquilizers in low doses. If the headache is associated with vomiting, an intramuscular injection of metoclopramide 2.0 ml is recommended. Isolated nausea and functional dyspepsia do not require pharmacological treatment.
6. For tremors, moderate convulsions or mild psychomotor agitation, tranquilizers are recommended. It is strongly advised not to use neuroleptics in these cases.
Interactions of benzylpiperazine with other substances, contraindications to usage
The “non-ADIOS" rule:
Non-Alcohol - it is not recommended using with alcohol.
Non-Dissociatives - it is not recommended using with dissociative drugs.
Non-iMAO - it is not recommended using with monoamine oxidase inhibitors.
Non-Opiates - it is not recommended using with opioid receptor agonists.
Non-Stimulators - it is not recommended using with stimulants.
Low risks when benzylpiperazine is used together with the following substances: benzodiazepines, cocaine, SSRI, MDMA, cannabis, caffeine.
Medium risk when benzylpiperazine is used together with the following substances: mushrooms, LSD, DMT, mescaline, 2С-х, ketamine, methoxetamine, alcohol, GHB
High risk: DOx, PCP, DXM, 5-MeO-xxT, 2C-Tx, NBOMes.
It is extremely dangerous to use benzylpiperazine together with αMT, tramadol, other opioid receptor agonists, MAO inhibitors.
Last edited by a moderator:
