G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,412
- Solutions
- 3
- Reaction score
- 2,379
- Points
- 113
- Deals
- 1
Introduction.
This file deals with the synthesis of GHB and related compounds. It is highly dangerous to attempt a synthesis of GHB without the proper knowledge of practical organic chemistry. The far most simple way to produce GHB is by the hydrolysis of the corresponding lactone (a cyclic intramolecular ester) to the desired hydroxy acid. Ester hydrolysis can be done in two ways: An acid catalyzed reaction or a base catalyzed reaction. The base catalyzed reaction is our choice here because the reaction is not reversible like the acid catalyzed one, and therefore we will get higher yields, and we will get the sodium salt of GHB, as the free acid is not stable, and will immediately cyclize into gamma-butyrolactone again.
Transformation Gamma-Butyrolactone to Sodium Gamma-Hydroxy Butyrate (Na-GHB).
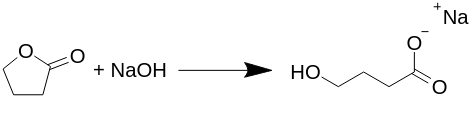
The reaction proceeds equimolarly (the same number of each molecule reacts), and there are no byproducts produced in this reaction, such as hydrogen gas, water, or anything else as proposed in several other texts. All published preparations of GHB, or more correctly Na-GHB, refluxes butyrolactone with sodium hydroxide in various solvents, usually in aqueous alcohol, but this is not necessary.
Physical/Chemical Properties.
gamma-Butyrolactone.
Mol wt 86.09; mp -43.53 °C; bp 204°C; d 1.12 g/ml
CAS No: [96-48-0]
Miscible with water, soluble in methanol, ethanol, acetone, ether, benzene
LD50: 1720 mg/kg (orally, mouse) 1540 mg/kg (orally, rat)
Uses: Solvent, paint remover, capacitor electrolyte, in organic chemistry
Synonyms: GBL, BLO, butyrolactone, gamma-hydroxy butyric acid lactone, 1,2-butanolide, 1,4-butanolide, 4-butanolide, 2-oxanolone, tetrahydro-2-furanone, dihydro-2(3H)-furanone.
Sodium GHB.
Mol wt 126.09; mp 145-146 °C
CAS No: [502-85-2]
LD50:2700 mg/kg (orally, rat)
Synonyms: Gamma-OH, sodium oxybate, sodium gamma-oxybutyrate, Somatomax PM, Wy-3478, NSC-84223, Somsanit, Anetamine.
Potassium GHB.
Mol wt: 142.20
Calcium GHB.
Mol wt 246.16; mp 164-166 °C, 166-168 °C.
Magnesium GHB.
Mol wt 230.39; mp (anhydrous) 172-174 °C; tetrahydrate 118-120 °C; pentahydrate 76-78 °C.
Mol wt 86.09; mp -43.53 °C; bp 204°C; d 1.12 g/ml
CAS No: [96-48-0]
Miscible with water, soluble in methanol, ethanol, acetone, ether, benzene
LD50: 1720 mg/kg (orally, mouse) 1540 mg/kg (orally, rat)
Uses: Solvent, paint remover, capacitor electrolyte, in organic chemistry
Synonyms: GBL, BLO, butyrolactone, gamma-hydroxy butyric acid lactone, 1,2-butanolide, 1,4-butanolide, 4-butanolide, 2-oxanolone, tetrahydro-2-furanone, dihydro-2(3H)-furanone.
Sodium GHB.
Mol wt 126.09; mp 145-146 °C
CAS No: [502-85-2]
LD50:2700 mg/kg (orally, rat)
Synonyms: Gamma-OH, sodium oxybate, sodium gamma-oxybutyrate, Somatomax PM, Wy-3478, NSC-84223, Somsanit, Anetamine.
Potassium GHB.
Mol wt: 142.20
Calcium GHB.
Mol wt 246.16; mp 164-166 °C, 166-168 °C.
Magnesium GHB.
Mol wt 230.39; mp (anhydrous) 172-174 °C; tetrahydrate 118-120 °C; pentahydrate 76-78 °C.
Laboratory procedures for the synthesis of GHB salts:
Please follow common Lab Safety procedures. Wear a lab coat and protective glasses. You will work with hot caustic solutions and solvents! Be aware of the risks associated with the manufacture of GHB! Never work alone!
Sodium GHB.
Procedure:
Dissolve 130 grams (3.25 moles) of pure sodium hydroxide in 400 ml of distilled water in a 1000 ml three-necked round-bottom flask while stirring. The dissolution is exothermic, and the solution will heat up. A cold water bath can be used to regulate the temperature. When everything has dissolved to form a clear solution, slowly add 250 ml (280 g, 3.25 moles) of gamma-butyrolactone in 50 ml portions with good stirring. The use of a dropping funnel is recommended. The addition of gamma-butyrolactone to the sodium hydroxide solution is also exothermic, and if it is added too fast, the solution will begin to boil, and we don't want that. Keep track of the temperature with an immersed thermometer. The addition of the gamma-butyrolactone will take somewhere between 20-30 minutes. When everything has been added, let the mixture react for an additional 10 minutes with occasional stirring.
Now it is time to see if the reaction has gone to completion by checking the pH with universal pH paper. We are aiming for a pH of 7-8. If it is too high (pH > 8), then add 10 ml of gamma-butyrolactone and let react for a few minutes more. If the pH is too low (pH < 7), add a few ml of concentrated NaOH aq. solution. Continue like this until the pH level is within the desired limits.
The solution is perfectly clear and tastes slightly salty. It may be slightly yellow colored, but not much if pure enough butyrolactone was used (distillation of the lactone before use takes care of this problem, b.p. 204 °C). If an acid is used to neutralize a too basic a solution (instead of adding more lactone), crystals of the sodium salt of the acid can precipitate in the solution, and the taste is severely impaired. The final solution will be around 750 ml, 50% NaGHB. The solution can be concentrated (by boiling off excess water) to ~600 ml without it crystallizing at room temp, but if concentrating as far as to ~500 ml it will invariably solidify.
Now it is time to see if the reaction has gone to completion by checking the pH with universal pH paper. We are aiming for a pH of 7-8. If it is too high (pH > 8), then add 10 ml of gamma-butyrolactone and let react for a few minutes more. If the pH is too low (pH < 7), add a few ml of concentrated NaOH aq. solution. Continue like this until the pH level is within the desired limits.
The solution is perfectly clear and tastes slightly salty. It may be slightly yellow colored, but not much if pure enough butyrolactone was used (distillation of the lactone before use takes care of this problem, b.p. 204 °C). If an acid is used to neutralize a too basic a solution (instead of adding more lactone), crystals of the sodium salt of the acid can precipitate in the solution, and the taste is severely impaired. The final solution will be around 750 ml, 50% NaGHB. The solution can be concentrated (by boiling off excess water) to ~600 ml without it crystallizing at room temp, but if concentrating as far as to ~500 ml it will invariably solidify.
Preparation of Sodium GHB using Sodium Bicarbonate (Baking Soda, NaHCO3).
Add 273 g NaHCO3 (3.25 moles) to 1125 mL distilled water in a three-necked round-bottom flask. Slowly bring the solution to a boil while stirring with a glass rod or similar. All the baking soda will dissolve. Carbon dioxide will be seen leaving the solution as it comes to a boil. This is the sodium bicarbonate breaking down into a slightly strong base, sodium carbonate:
Reduce the heat to a light boil, and slowly add 250 ml gamma-Butyrolactone (280 g, 3.25 moles). The addition is not immediately exothermic, as with the sodium hydroxide synthesis. Keep this solution at a light boil for 30 minutes. Check the pH with universal pH paper. We are aiming for a pH around 7, but anything 6 to 8 is perfectly safe. If the pH is too high, add a small amount more GBL and continue to reflux.
The solution will be perfectly clear and should be absolutely colorless. If it is not perfectly colorless, i.e. if slightly impure butyrolactone was used and the solution has taken on a light yellow color, add about 100 mL of activated charcoal. Allow this to boil for 10 minutes. Cool the solution, then filter, washing the activated charcoal two or three times with 50 ml portions of cold water. 410 g of NaGHB will be made in this synthesis. This solution can be concentrated to about 50% NaGHB before it will start to crystallize. If you wish for a powder, heat until the temperature of the solution reaches 150 °C, then pour onto a cooled Pyrex dish and allow it to cool and solidify. This synthesis is perfect for use where there is no ACS, Food or Electronics grade sodium hydroxide available.
The solution will be perfectly clear and should be absolutely colorless. If it is not perfectly colorless, i.e. if slightly impure butyrolactone was used and the solution has taken on a light yellow color, add about 100 mL of activated charcoal. Allow this to boil for 10 minutes. Cool the solution, then filter, washing the activated charcoal two or three times with 50 ml portions of cold water. 410 g of NaGHB will be made in this synthesis. This solution can be concentrated to about 50% NaGHB before it will start to crystallize. If you wish for a powder, heat until the temperature of the solution reaches 150 °C, then pour onto a cooled Pyrex dish and allow it to cool and solidify. This synthesis is perfect for use where there is no ACS, Food or Electronics grade sodium hydroxide available.
Potassium GHB.
Use the ethanol synthesis described above for sodium GHB, but substitute the 130 grams of NaOH for 182 grams of KOH (This calculation is based on the heavier K atom, and the higher water content of KOH versus NaOH). Using KOH gives users of K-GHB that Potassium supplement that is by some said to be needed in connection with administration of GHB. Bear in mind that (powdered) K-GHB is slightly less active (by weight) than Na-GHB, as the K ion is heavier than the Na counterpart. Differences between K-GHB and Na-GHB is that the K salt is more soluble in water than the Na salt, and the taste is more like salt/licorice instead of the salt/soap taste of Na-GHB.
Calcium GHB.
74 g analytically pure calcium hydroxide are suspended in 200 ml of distilled water. 160 ml 4-butyrolactone are added in portions (each portion about 5 to 10 ml) and under stirring to this suspension at room temperature. After addition of 20 ml, the reaction mixture warms to about 50 °C to 60 °C. The addition of 4-butyrolactone is controlled such that the temperature remains between about 50 °C and 60 °C, which takes about 1 hour. During this time, the calcium hydroxide has dissolved practically completely. The reaction material is contaminated with a slight rust-yellow precipitate. It is thinned down with 300 ml methanol, is left for four hours to itself and is then filtered through a folded filter. The clear filtrate is cautiously treated with 200 ml acetone in the way that after each portion of acetone causing a precipitate, time is allowed for the precipitate to redissolve. A water clear solution is obtained, which is placed for crystallization. After two hours of standing, colorless crystals start to deposit. In this state, the crystallization is accelerated by continuous addition of acetone (in total 100 ml). The crystallization time is 24 hours. The crystals are sucked off and are washed initially with 50 ml methanol and then additionally with 60 ml acetone. The crystals are dried at temperatures from about 60 °C to 80 °C. in a drying cabinet. Yield: 230 g. Melting point 166-168 °C. (immediately). The product is the water-free nonhygroscopic calcium salt of the 4-hydroxybutyric acid. It is dissolvable as desired in water, the aqueous solution has a pH- value of 7 to 7.5. The salt can be stored as long as desired and does not change in air. Even upon storage, no water is attracted from the air.
The residue crystallizes to a mass of colorless crystals, which is after dried at temperatures from about 60 °C to 80 °C. Yield: about 105 g. Melting point 164-166 °C. The product is Di-(4-hydroxybutyric) calcium. It is recrystallized by dissolving in a little methanol, followed by adding of acetone to cloudiness, and crystallizing in the cold.
Instead of methanol also ethanol and isopropanol can be employed for recrystallization with the same success. Without employing water containing alcohols as recrystallization medium or as additive of the recrystallization and purification, no stable and in particular no nonhygroscopic calcium salts are obtained. The water content of the alcohols should be from about 3-10% by volume. The such obtained final product does easily dissolve in water, is not hydroscopic and has a pleasant aromatic odor.
The residue crystallizes to a mass of colorless crystals, which is after dried at temperatures from about 60 °C to 80 °C. Yield: about 105 g. Melting point 164-166 °C. The product is Di-(4-hydroxybutyric) calcium. It is recrystallized by dissolving in a little methanol, followed by adding of acetone to cloudiness, and crystallizing in the cold.
Instead of methanol also ethanol and isopropanol can be employed for recrystallization with the same success. Without employing water containing alcohols as recrystallization medium or as additive of the recrystallization and purification, no stable and in particular no nonhygroscopic calcium salts are obtained. The water content of the alcohols should be from about 3-10% by volume. The such obtained final product does easily dissolve in water, is not hydroscopic and has a pleasant aromatic odor.
Magnesium GHB.
60 g magnesium hydroxide (analytical grade) are suspended in 200 ml tap water under stirring. In a stream and under stirring 160 ml butyrolactone are mixed into this suspension. Then the mixture is heated on a water bath for 6 hours under stirring in a 2-liter-flask. The magnesium hydroxide dissolves practically completely. The flask is allowed to stand overnight, while contaminants deposit and the solution is decanted without effort from the contaminant deposit. The water clear decantate is initially stirred with 100 ml acetone for 10 minutes. The colorless syrupy liquid, which now turned more viscous, is mixed again with 100 ml acetone as described above, the acetone is again removed by decanting and the fairly viscous, colorless syrup is left to itself at room temperature for about 2 to 4 hours. It solidifies to a colorless crystal mass, which is comminuted in a mortar and dried for several hours in air. Melting point 76 °C to 78 °C. Yield: 314 g in analytically pure form.
This magnesium salt contains about 5 moles of water of hydration. It is not hydroscopic, is stable and can be stored for arbitrary long times. By drying over several hours at 40 °C to 50 °C, it loses part of its water (1 mole) of crystallization and then melts at 118 °C to 120 °C. Water-free magnesium 4-hydroxybutyrate can be produced by removal of water by sublimation and/or evaporation of water under decreased partial pressure of water and at elevated temperature, or by crystallization from a solution containing an organic solvent. The water-free salt melts at 172-174 °C. The chemical analysis shows 10.50 weight percent magnesium (calculated 10.55% weight magnesium). All modifications are nonhygroscopic and stable during storage. 1 g of the magnesium salt dissolves in 2 ml water at room temperature, the pH of the aqueous solution is 7. It dissolves easily in water, methanol and ethanol, it does not dissolve in ether and hydrocarbons, it is not hygroscopic, is storable and has a pleasant aromatic odor.
This magnesium salt contains about 5 moles of water of hydration. It is not hydroscopic, is stable and can be stored for arbitrary long times. By drying over several hours at 40 °C to 50 °C, it loses part of its water (1 mole) of crystallization and then melts at 118 °C to 120 °C. Water-free magnesium 4-hydroxybutyrate can be produced by removal of water by sublimation and/or evaporation of water under decreased partial pressure of water and at elevated temperature, or by crystallization from a solution containing an organic solvent. The water-free salt melts at 172-174 °C. The chemical analysis shows 10.50 weight percent magnesium (calculated 10.55% weight magnesium). All modifications are nonhygroscopic and stable during storage. 1 g of the magnesium salt dissolves in 2 ml water at room temperature, the pH of the aqueous solution is 7. It dissolves easily in water, methanol and ethanol, it does not dissolve in ether and hydrocarbons, it is not hygroscopic, is storable and has a pleasant aromatic odor.
Other salts.
The Lithium and Ammonium salts of GHB would be dangerous to ingest. Lithium-ion is toxic, and together with NH3 lactone becomes pyrrolidone.
Synthesis Q&A.
Q: Can I use lye instead of pure sodium hydroxide?
A: No, that could have unpredictable results on your health. Hardware store lye does not have anywhere near the rigorous purity criterions of for example food grade, ACS grade or electronics grade. Some people tell about successful stories using lye, which really is possible, but as said, the results are unpredictable.
Q: I do not have the glassware you say are needed, can I boil the solution in a pot on the stove instead?
A: No, you can not. The sodium hydroxide will corrode the metal, and assorted metal ions will get into your product. You can, of course, use simpler glassware than in my suggestions, and make the necessary adjustments of the procedure.
Q: I cannot recrystallize the Na-GHB from ethanol. It forms a sticky mess.
A: Your Na-GHB is not dry, or your ethanol is not anhydrous. Water makes the recrystallization almost impossible. The fact that the sodium GHB is deliquescent (hygroscopic) does not make this better. You must dry the GHB thoroughly, preferably in a vacuum desiccator before attempting recrystallization, or any other improvised alternative. The ethanol you are planning to use (most often supplied in a purity of 95%, the rest being water) must be dried by drying over anhydrous calcium sulfate followed by distillation from calcium oxide with adequate measures taken to exclude moisture from the reaction.
Q: Where can I buy butyrolactone/Is it safe to buy butyrolactone?
A: I have no idea how the situation is for you in your country. The answers to these questions highly depends on who you are and where you live. However, you can contact any of the many sellers of GHB Kits which can be found online.
A: No, that could have unpredictable results on your health. Hardware store lye does not have anywhere near the rigorous purity criterions of for example food grade, ACS grade or electronics grade. Some people tell about successful stories using lye, which really is possible, but as said, the results are unpredictable.
Q: I do not have the glassware you say are needed, can I boil the solution in a pot on the stove instead?
A: No, you can not. The sodium hydroxide will corrode the metal, and assorted metal ions will get into your product. You can, of course, use simpler glassware than in my suggestions, and make the necessary adjustments of the procedure.
Q: I cannot recrystallize the Na-GHB from ethanol. It forms a sticky mess.
A: Your Na-GHB is not dry, or your ethanol is not anhydrous. Water makes the recrystallization almost impossible. The fact that the sodium GHB is deliquescent (hygroscopic) does not make this better. You must dry the GHB thoroughly, preferably in a vacuum desiccator before attempting recrystallization, or any other improvised alternative. The ethanol you are planning to use (most often supplied in a purity of 95%, the rest being water) must be dried by drying over anhydrous calcium sulfate followed by distillation from calcium oxide with adequate measures taken to exclude moisture from the reaction.
Q: Where can I buy butyrolactone/Is it safe to buy butyrolactone?
A: I have no idea how the situation is for you in your country. The answers to these questions highly depends on who you are and where you live. However, you can contact any of the many sellers of GHB Kits which can be found online.
Precursors.
The obvious precursor for the synthesis of GHB is gamma-Butyrolactone. It can be made from pre-precursors such as Tetrahydrofuran (THF) with oxidants such as Ruthenium tetroxide, calcium hypochloriteand nitric acid. 4-Halo-butyric acid derivatives (chloro, bromo, iodo) can also be used. As in the synthesis below, they can be converted to gamma-butyrolactone by distillation with sodium methoxide.
gamma-Butyrolactone from 4-bromobutyric acid.
To a solution of 7.8 g of sodium in 500 ml of absolute alcohol was added 60.5 g of 4-bromobutyric acid. The reaction mixture was boiled under a reflux condenser for about five hours. During this time, sodium bromide separated. The alcohol was distilled from a steam bath, and the lactone was separated from the sodium bromide by extraction with ether. The ether was evaporated, and the lactone distilled under ordinary pressure. The yield was 21.2 grams (67%) of product boiling at 202-206 °C. An alternative may be free radical chlorination of butyric acid with sulfur chloride in the presence of peroxides, and separate the isomers through distillation, make the sodium salt of 4-chlorobutyric acid, and cyclize to the lactone as with the 4-bromo derivative above. gamma-Butyrolactone can also be made from 4-methoxybutyric acid, 3-phenoxypropylcyanide, gamma-diethylaminobutyric acid and beta-chloro ethyl vinyl ether as well as many other a bit too exotic chemicals. Industrially, it is commonly made by reacting acetylene with formaldehyde under high temperatures and pressures.
Dehydrogenation of 1,4-Butanediol (BDO) to gamma-Butyrolactone (GBL).
A mixture of 90.1 g (1 mole) 1,4-butanediol, 4 g of copper chromite catalyst and 0.15 g of powdered reagent grade sodium hydroxide (or better KOH) was stirred vigorously and heated under reflux. At about 200 °C, a lively evolution of hydrogen occurred, and the temperature dropped about 10 °C and the dehydrogenation proceeded smoothly. The evolution of gas (39 L/2 mol per mole 1,4-butanediol reacted) ceased in about 3 h. The reaction mixture was cooled to room temperature, filtered from the catalyst and distilled under reduced pressure to give gamma-butyrolactone in about 80% yield and unreacted 1,4-butanediol in about 10% yield.
Preparation of high-active copper chromite dehydrogenation catalyst.
A solution of 260 g of Copper(II)nitrate trihydrate in 900 ml of tap water at 80 °C was added while stirring to a soln 178 g of sodium dichromate dihydrate and 225 ml of 28% NH4OH made up to 900 ml at 25 °C. The precipitate was collected by suction filtration and slurried in water three times. The copper ammonium chromate was dried at 75-80 °C overnight. This was powdered and added in small portions to a one liter 3-neck flask equipped with a stainless-steel stirrer Hershberg type which scrapped close to the bottom of the flask. The flask was partially immersed in an oil bath at 350 °C (with 300-320 °C, one can obtain good results). The time of addition was 15 min (a lot of fumes evolved) and the mixture was stirred at 350 °C for another 15 min after all was complete. The cooled, and black dusty powder used as is for the dehydrogenation.
A solution of 260 g of Copper(II)nitrate trihydrate in 900 ml of tap water at 80 °C was added while stirring to a soln 178 g of sodium dichromate dihydrate and 225 ml of 28% NH4OH made up to 900 ml at 25 °C. The precipitate was collected by suction filtration and slurried in water three times. The copper ammonium chromate was dried at 75-80 °C overnight. This was powdered and added in small portions to a one liter 3-neck flask equipped with a stainless-steel stirrer Hershberg type which scrapped close to the bottom of the flask. The flask was partially immersed in an oil bath at 350 °C (with 300-320 °C, one can obtain good results). The time of addition was 15 min (a lot of fumes evolved) and the mixture was stirred at 350 °C for another 15 min after all was complete. The cooled, and black dusty powder used as is for the dehydrogenation.
gamma-Butyrolactone Synthesis from Tetrahydrofuran (THF).
To a stirred mixture of 7.2 g (0.1 mol) tetrahydrofuran in 100ml water there was added 15.1 g of sodium bromate and 13.6 g (0.1 mol) potassium hydrogen sulfate. External cooling was necessary to keep the temperature of the solution between 25-30 °C. Stirring was continued for 16 hours at room temperaturem, after which time all the THF had been consumed. To quench any excess bromine formed, acidic sodium sulfite solution was used [bisulfite should work just as good]. Thus, 140-150 ml of a 10% solution was added and 13.6 g (0.1 mol) potassium hydrogen sulfate. The reaction was cooled and extracted with 5x30 ml dichloromethane. The combined organic layers was dried over MgSO4 and the solvent removed under vacuum. The residue was distilled to give gamma-butyrolactone in 73% yield (bp 204-205 °C).
Sandmeyer Reaction of GABA to GBL/GHB.
It is awesome for a chemist who wishes to prepare GHB in small quantities and high yields and to do so without directly obtaining any regulated chemicals such as gamma-butyrolactone (GBL) or 1,4-butanediol (BDO). It also avoids the typically low yields seen from the oxidation of tetrahydrofuran (THF). It uses an easy to obtain amino acid, gamma-aminobutyric acid (GABA), and sodium nitrite (NaNO2). It scales very nicely and runs without too much hassle. Not one suspect chemical is used.
The Sandmeyer reaction uses nitrous acid to turn amines into diazonium salts. This reaction, as it applies to turning GABA into GHB, is shown in the first reaction below. Aliphatic diazonium salts rapidly undergo hydrolysis in the presence of water giving off nitrogen gas and leaving a hydroxyl group behind. This is shown in the second step. As a result of these reactions, GABA can be turned into GHB in an easy to perform one-pot reaction.
Running the reaction.
Set up a 2 L flask, sitting in ice-water on top of a magnetic stirrer. Now:
Running the reaction.
Set up a 2 L flask, sitting in ice-water on top of a magnetic stirrer. Now:
- Add 3mol GABA (309.4 g).
- Add 3mol NaNO2 (207.0 g).
- Add 700ml water (total volume becomes about 1100 ml).
- Drop in a 1" stir bar and start stirring.
- Charge a 500 ml pressure equalized addition funnel with 3.3 mol HCl(aq) (385.0 g 31.25%, 334.8 ml 31.25%).
- Fit the addition funnel with a gas outlet adapter and vent to the outside.
Begin slowly dripping the hydrochloric acid into the mixture. Drip it in at a constant rate of about 1 drop every 2-5 seconds. Speed it up as time progresses and replace the ice as necessary, but do not allow the evolution of the brown poisonous gas to become vigorous. After about one hour after the last drop of acid has been added, there is no need to replace the ice. Once the reaction is done, proceed to extract. (usually 24-36 hours later)
Extraction.
There are many options for this. This is still a work in progress, but after about 20 runs, I came to use this work up. You can use ethyl acetate (EtOAc), chloroform or methylene chloride (dichloromethane aka DCM) to perform the solvent extractions. I have normally used DCM as it's nice since the organic layer drops to the bottom of the separatory funnel.
1. Setup for a simple distillation
Extraction.
There are many options for this. This is still a work in progress, but after about 20 runs, I came to use this work up. You can use ethyl acetate (EtOAc), chloroform or methylene chloride (dichloromethane aka DCM) to perform the solvent extractions. I have normally used DCM as it's nice since the organic layer drops to the bottom of the separatory funnel.
1. Setup for a simple distillation
a) Distill, throwing out the first 5-10 mls, or so, of distillate as it will contain a fair amount of nitric oxides. Distill off as much water as possible, basically until the sodium chloride starts to saturate the aqueous layer and precipitate out.
b) The remainder of the distillate (approximately 700 ml) will contain approximately 1 g GBL/10 ml.
c) Treat the remainder of the distillate with NaHCO3 at reflux for 30 mins.
d) Boil with about a 5% volume of activated charcoal (i.e .35 ml activated charcoal) (compared to the volume of the solution) for 5-10mins.
e) Allow it to cool and filter, wash the charcoal with distilled water. Save the NaGHB.
2. With the remainder of the aqueous, extract 5 times with 625ml portions of DCM.
3. Distill off the DCM (reuse that DCM!).
4. Distill the GBL (under vacuum if available).
5. React with NaHCO3 and distilled water and treat with activated charcoal as before.
Typically, 375 g of NaGHB is made from the solvent extracted GBL of which 100 g NaGHB from the aqueous distillate. Although conversion is nearly quantitative (as measured by GC/MS), the overall recovered yield is usually about 70%.
3. Distill off the DCM (reuse that DCM!).
4. Distill the GBL (under vacuum if available).
5. React with NaHCO3 and distilled water and treat with activated charcoal as before.
Typically, 375 g of NaGHB is made from the solvent extracted GBL of which 100 g NaGHB from the aqueous distillate. Although conversion is nearly quantitative (as measured by GC/MS), the overall recovered yield is usually about 70%.
Last edited:
