G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,791
- Solutions
- 3
- Reaction score
- 3,047
- Points
- 113
- Deals
- 1
Introduction

In recent years the production of synthetic drugs such as ecstasy, amphetamine and other amphetamine type stimulants has dramatically increased. Although the number of underground laboratories discovered in the European Union has stabilised, the professionalism and production capacity of such sites has increased significantly, not only for the production of synthetic drugs but also in the production of precursors and reductors. A synthetic drug is the end product of a chemical process, as indicated by the word “synthesis” meaning: A reaction between two or more chemicals. Synthetic drugs are therefore chemical products dependent in the required chemicals for their production or the availability of such chemicals. Legitimate chemical processes are undertaken in specially created environments, such as chemical factories, using highly sophisticated equipment, pure chemicals and the necessary chemical knowledge where, even then, chemical waste will be an unavoidable by product. During the production of synthetic drugs, huge amounts of waste will result.
The production of 1 kg amphetamine or ecstasy will, depending on the production method used, result in 5 to 20 litres of waste (i.e. the Leuckart synthesis produces more waste than reductive amination). Furthermore, during specific steps of the production process, certain amounts of solvents will vaporise and thereby pollute the atmosphere. Chemical waste is a combination of the chemicals used, the by-products and the end products. As more than 200 different chemicals can be used in synthetic drug production, the resultant waste will vary significantly in terms of content and hazardous properties such as flammability, explosiveness, toxicity, corrosiveness, oxidation, carcinogens and others.
The production of 1 kg amphetamine or ecstasy will, depending on the production method used, result in 5 to 20 litres of waste (i.e. the Leuckart synthesis produces more waste than reductive amination). Furthermore, during specific steps of the production process, certain amounts of solvents will vaporise and thereby pollute the atmosphere. Chemical waste is a combination of the chemicals used, the by-products and the end products. As more than 200 different chemicals can be used in synthetic drug production, the resultant waste will vary significantly in terms of content and hazardous properties such as flammability, explosiveness, toxicity, corrosiveness, oxidation, carcinogens and others.
The “quality” of the waste will differ, depending on the following circumstances:
- The production processes used.
- The quality of the chemicals and equipment used.
- The knowledge and relative efficiency of the chemist and his methods.
- The chemical mixture ratio; i.e. if excess chemical is added to a process, the surplus will be converted into chemical waste which must be removed.
- The mixture of different waste products; i.e. individual production steps result in different waste which might be mixed and stored together.
Types of laboratory waste
In most cases, the chemical waste content exists of one or more of the following chemicals:Solids
This type of laboratory waste represents such as solid packages (paper, plastic), damaged glassware.Liquids
This type of laboratory waste are widely produced after synthesis of drugs. Different solvents such as acetone, ether, methanol, iso-propanol, toluene formamide, ammonia; Acids: sulphuric, hydrochloric, phosphorus and so on.Bulk
Slags, caustic soda, residues of benzylmethylketone, piperonylmethylketone, iso-safrol, etc.Gaseous
Gas wastes like NO2, HCl, Cl2, Br2, vapors of solvents, Hg vapors may be removed by good filter-tipped ventilation (pull out drobe, exhaust hood).Recuperation of solvents
Solvent recovery is a form of waste reduction. In-process solvent recovery is widely used as an alternative to solvent replacement to reduce waste generation. It is attractive, like end-of-pipe pollution control, since it requires little change in existing processes. Availability of equipment suitable for small operations, especially batch operations, make in-process recovery of solvents economically preferable to raw materials' substitution. Commercially available solvent recovery equipment includes:
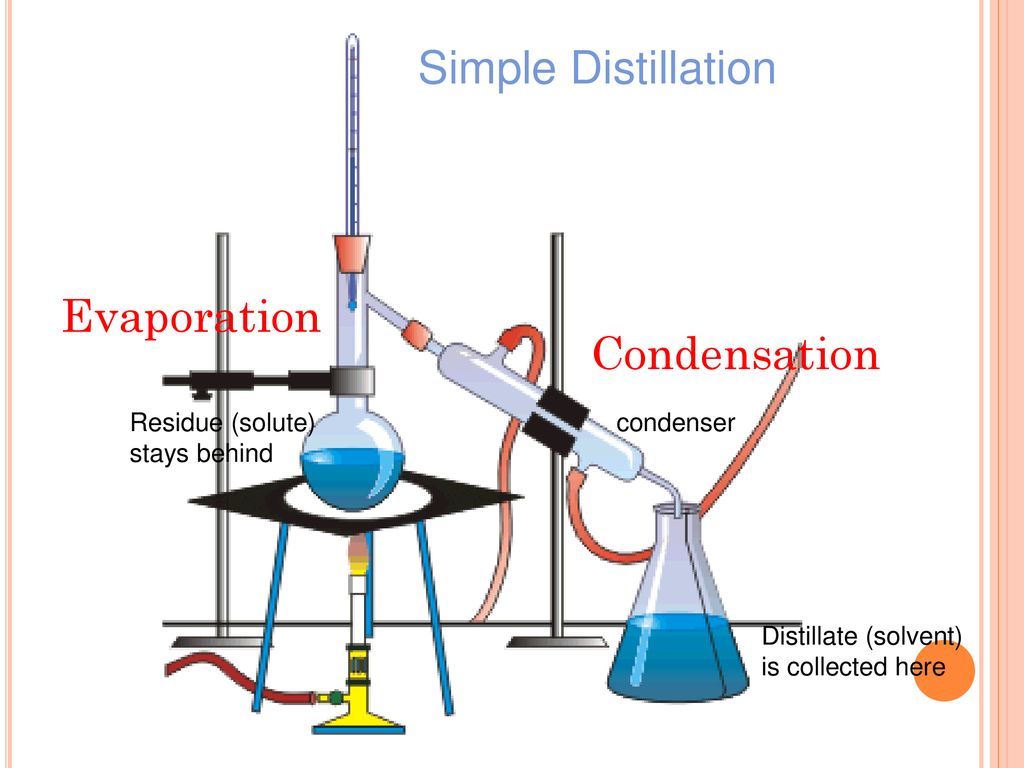
- Distillation and condensation can be used to separate and recover solvent from other liquids. Removal efficiency can be very high using this process and can be used for solvent mixtures as well as single solvents. It is the most effective method for drug manufacturing laboratory. You have to use detached distillation apparatus for this procedure. This procedure saves a lot of solvent and money eventually.
- Carbon adsorption of solvent, removal of the solvent by steam, and separation of the solvent for reuse in the operation. Carbon must be regenerated, two or more units are required to keep the operations continuous. Chloric acid formation from chlorinated solvents, carbon bed plugging by particulates, and buildup of certain volatile organics on the carbon and corrosion can be a problem. Also, you can add activated carbon in your solvent or solvent mix before distillation to purify them from some dissolved substances (in this case, use activated carbon once).
- Dissolving the solvent in another material, such as scrubbing. Solvents must be then recovered from the resulting solution, through distillation, but efficiency of removal is often not high using this method.
Storage of chemical waste
Such chemical waste is typically stored in old jerry cans, barrels and other means of storage. Any container used to store hazardous waste must be in good condition (tight-fitting lids, no leads, no corrosion, no rust, cracks, or serious dents). The collection container must be labelled as soon as collection begins. At a minimum, the container must be labelled with the fully written, proper chemical name of the material contained within, and wording to indicate if the chemicals are used, spent, excess, etc. If a container is a mixture of chemicals, the container must be labelled with the names of all components in the mixture. Be sure that the container is compatible with the chemical waste. Use containers that are made of or lined with materials which will not react with, and are otherwise compatible with, the hazardous waste to be stored. The following table shows general chemical categories and compatible container type.
Chemical Category — Container Type
Mineral acids — Plastic
Bases — Plastic
Oxidizers — Glass
Organics, including acetic acid — Glass
Mineral acids — Plastic
Bases — Plastic
Oxidizers — Glass
Organics, including acetic acid — Glass
Waste containers must be closed at all times, except when being filled. Do NOT leave funnels in the containers. Do NOT place any types of vials inside a waste container. There is a separate waste container for glass items and sharps. Only chemical waste can be disposed of in waste containers. Do not accumulate excessive waste in the work area. The laboratory should NOT have more than one container of each kind of waste. Be sure that containers in the waste storage area do not leak. Consider the use of secondary containment, such as a tray or larger container, to prevent a possible spill or leak from the waste containers. Like any chemical storage in the laboratory or work area, be sure to segregate the containers according to the type of waste. Chemical wastes that are incompatible should not be stored together. If these wastes must be stored in the same area, they should be physically separated to prevent the materials from contacting each other in the event of a spill or leak.
NEVER store the following types of wastes near each other:
- Organics and acids
- Cyanide, sulfide, or arsenic compounds and acids
- Alkali or alkali earth metals, alkyl-lithiums, etc. and aqueous waste
- Powdered or reactive metals (for example Mg) and combustible materials
- Mercury or silver and ammonium containing compounds
Broken glassware are usually collected in plastic-lined cardboard boxes for landfilling. Due to contamination, they are frequently not suitable for recycling. Waste elemental mercury, spent acids and bases may be collected separately for recycling.
Disposal of chemical waste
There are several ways:
- Heat treatment: combustion or pyrolysis (carried out without access to oxygen). Waste incinerators are used. Alternatives include autoclaving, steaming and microwave ovens to minimize environmental damage due to the release of hazardous substances.
- Neutralization of laboratory waste at the place of generation.
- Distillation applied for the purpose of subsequent use (solvents).
- Recycling - for example, chemical neutralization (acids, alkalis).
- Burial at landfills.
Innocuous aqueous waste (such as solutions of sodium chloride) may be poured down the sink. Some chemicals are washed down with excess water. This includes concentrated and dilute acids and alkalis, harmless soluble inorganic salts (all drying agents), alcohols containing salts, hypochlorite solutions, fine (tlc grade) silica and alumina. Aqueous waste containing toxic compounds are collected separately.
Do not dispose the acidic waste down the sewer directly. Dilute with tap water and pour into the plastic container, containing marble chips. The dilution is facilitated by running tap water at the same time as the acid (or base). For example, a faucet running at a rate of 1 liter per minute produces a 1:10 dilution of 1 -liter of concentrated acid over 10 minutes. The acidic solution after coming in contact with the marble chips will neutralize it, after dilution, and the fluid will drain out into the outer container, that has additional drains, through which, the fluid, drains out into the sink drain and down the sewer. This bucket is referred to as the “neutralization bucket” in the laboratory. Doing a dilution and neutralization in the sink will simplify disposal of acidic solutions.
Do not dispose the acidic waste down the sewer directly. Dilute with tap water and pour into the plastic container, containing marble chips. The dilution is facilitated by running tap water at the same time as the acid (or base). For example, a faucet running at a rate of 1 liter per minute produces a 1:10 dilution of 1 -liter of concentrated acid over 10 minutes. The acidic solution after coming in contact with the marble chips will neutralize it, after dilution, and the fluid will drain out into the outer container, that has additional drains, through which, the fluid, drains out into the sink drain and down the sewer. This bucket is referred to as the “neutralization bucket” in the laboratory. Doing a dilution and neutralization in the sink will simplify disposal of acidic solutions.
Stages:
1. Waste collection inside the laboratory.
2. Separation of waste by type. Part of the waste that can be poured into the sink is neutralized and poured into the sink. The part that cannot be neutralized or thrown down the sink (immiscible solvents, solid waste, etc. see above) is incinerated or landfilled.
3. Delivery to the area of burial/destruction.
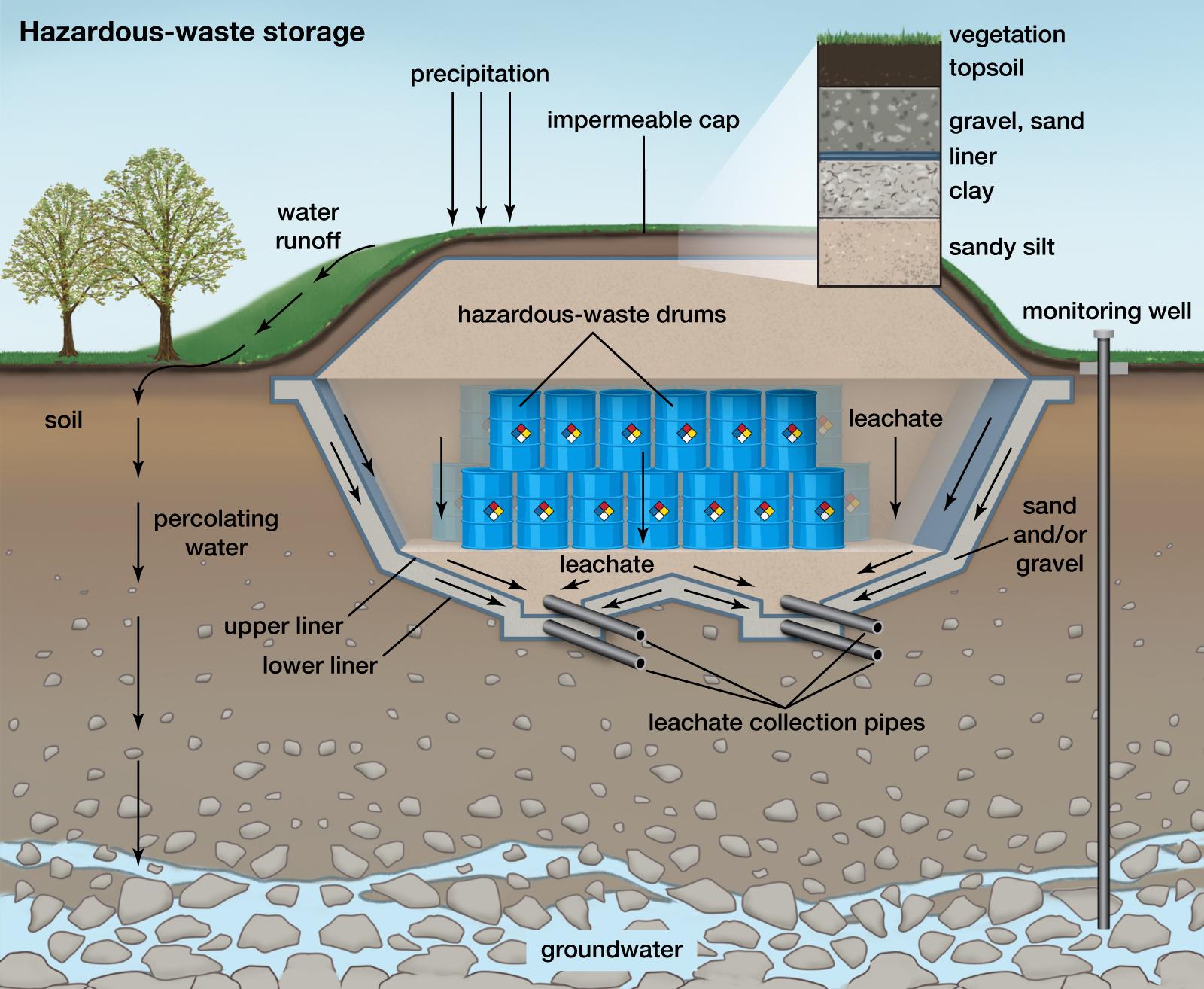
You must avoid contaminants entering the soil and groundwater. Take care to carefully isolate your burials from them, as shown in the picture, or close to it.
2. Separation of waste by type. Part of the waste that can be poured into the sink is neutralized and poured into the sink. The part that cannot be neutralized or thrown down the sink (immiscible solvents, solid waste, etc. see above) is incinerated or landfilled.
3. Delivery to the area of burial/destruction.
You must avoid contaminants entering the soil and groundwater. Take care to carefully isolate your burials from them, as shown in the picture, or close to it.
Incineration of waste
For waste incineration, you can use a small and inexpensive portable batch incinerator. Twin 110V electric high velocity blowers create a cyclone of intense heat generating temperatures high enough that it burns clean and smoke-free! It can be used with or without fuel. For dry loads, that support combustion, it incinerates waste with incredible efficiency. For loads with a moisture content above 15%, the unique optional fuel injection system allows the incineration of a large variety of refuse and waste materials. Combustion is so complete, the volume of materials is reduced to an average of 3% ash. Portable and convenient, it stores easily and rolls out of your way when the job is done.
In case of lack of money or you have some wastes, you can use simple old barrel with duct hole in the close bottom. The disadvantage of this method is the amount of fume. Nevertheless, you can move it in safety place to burn your wastes.
Conclusion
Waste disposal is an essential stage of the underground laboratory. Some mistakes can lead to arrest and loss of everything. You have to take care of timely disposal of waste. I strongly recommend you to recuperate solvents by distillation because it decreases cost of your product and increase your benefits. Although you need to buy some things for distillation of waste solvents, in has a good benefit in long run and will reduce the amount of waste in the production of your product.
Last edited by a moderator:
