- Language
- 🇬🇧
- Joined
- Dec 2, 2023
- Messages
- 316
- Reaction score
- 29
- Points
- 28
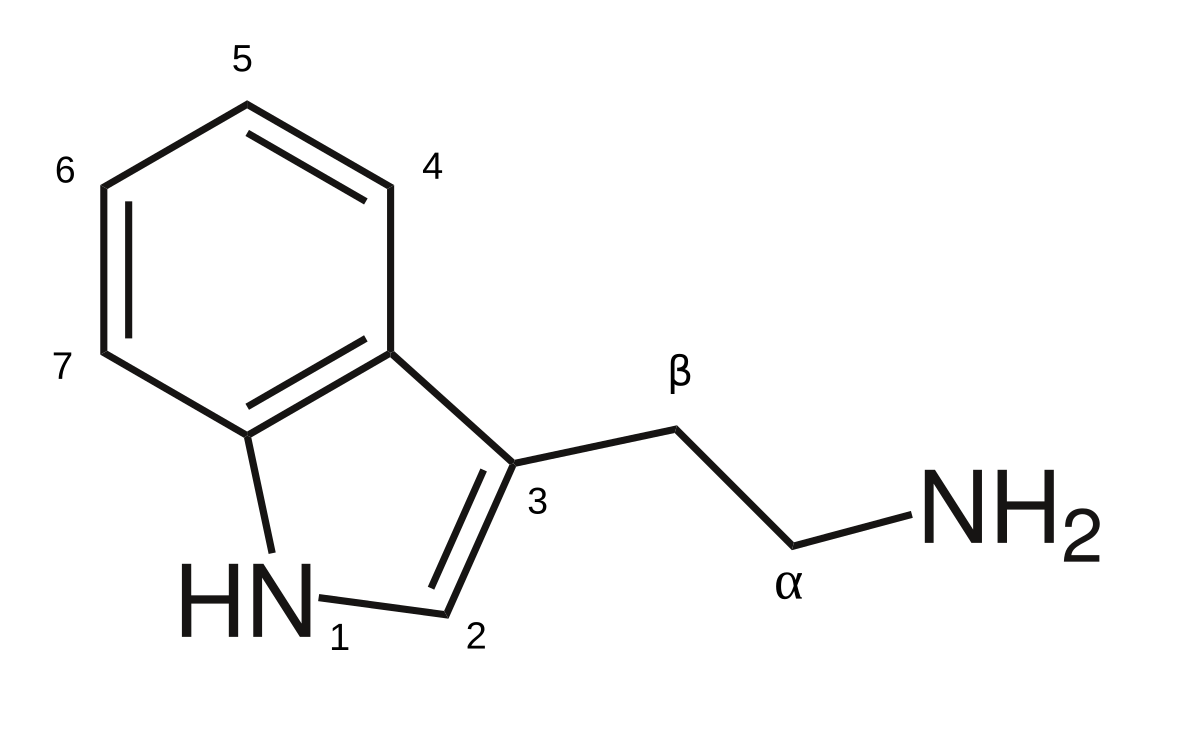
Okay so I’m sure some people are aware iv been making,n-DMT and other tryptamines using reductive amination at cold temps…
so I’m wondering, seeing as there are so many things based on the 2,5-dimethoxybenzaldehyde, I’m curious what if you were to use that as the source of aldehyde in this reaction?
i also wondered the same of cyclohexanone but as a ketone of course…
so with the former would you hypothetically obtain something like 2,5-dimethoxy-4-methyltryptamine? Would this be active?
how about n-cyclohexyltryptamine?
are any of these likely to be active at all? I need to try and do more general reading on structure activity relationships, which is my plan over the weekend on a couple subjects, this being one of them….
I guess it just got me thinking….
so I’m wondering, seeing as there are so many things based on the 2,5-dimethoxybenzaldehyde, I’m curious what if you were to use that as the source of aldehyde in this reaction?
i also wondered the same of cyclohexanone but as a ketone of course…
so with the former would you hypothetically obtain something like 2,5-dimethoxy-4-methyltryptamine? Would this be active?
how about n-cyclohexyltryptamine?
are any of these likely to be active at all? I need to try and do more general reading on structure activity relationships, which is my plan over the weekend on a couple subjects, this being one of them….
I guess it just got me thinking….