G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,436
- Solutions
- 3
- Reaction score
- 2,432
- Points
- 113
- Deals
- 1
Introduction
There are two effective routes to obtain phenylacetic acid from benzene chloride. First way via benzyl cyanide allow getting up to 77% yield. The second way from benzyl chloride via Grignard reagent describes to 60% yield from theoretical. Both ways have some advantages and disadvantages. For instance, benzyl cyanide synthesis takes a lot of experience from a chemist because he has to work with sodium cyanide, which is very toxic. In the other hand, the way from benzyl chloride via Grignard reagent take less toxic reagents, but chemist have to work with solid carbon dioxide or Kipp's apparatus. Also, this method give less yield than first one. In our forum you can find other ways of phenylacetic acid synthesis such as route from Mandelic acid or Benzyl cyanide via alkaline hydrolysis.
Moreover, in this topic you can find detailed benzyl chloride synthesis from benzene. In the end, I want to warn any chemists, who want to repeat this synthesis, that you have to use all safety equipment such as chemical glass, gloves, chemical coat and respiratory mask. Carry on experiments under pull out probe or under exhaust hood in good ventilated premises.
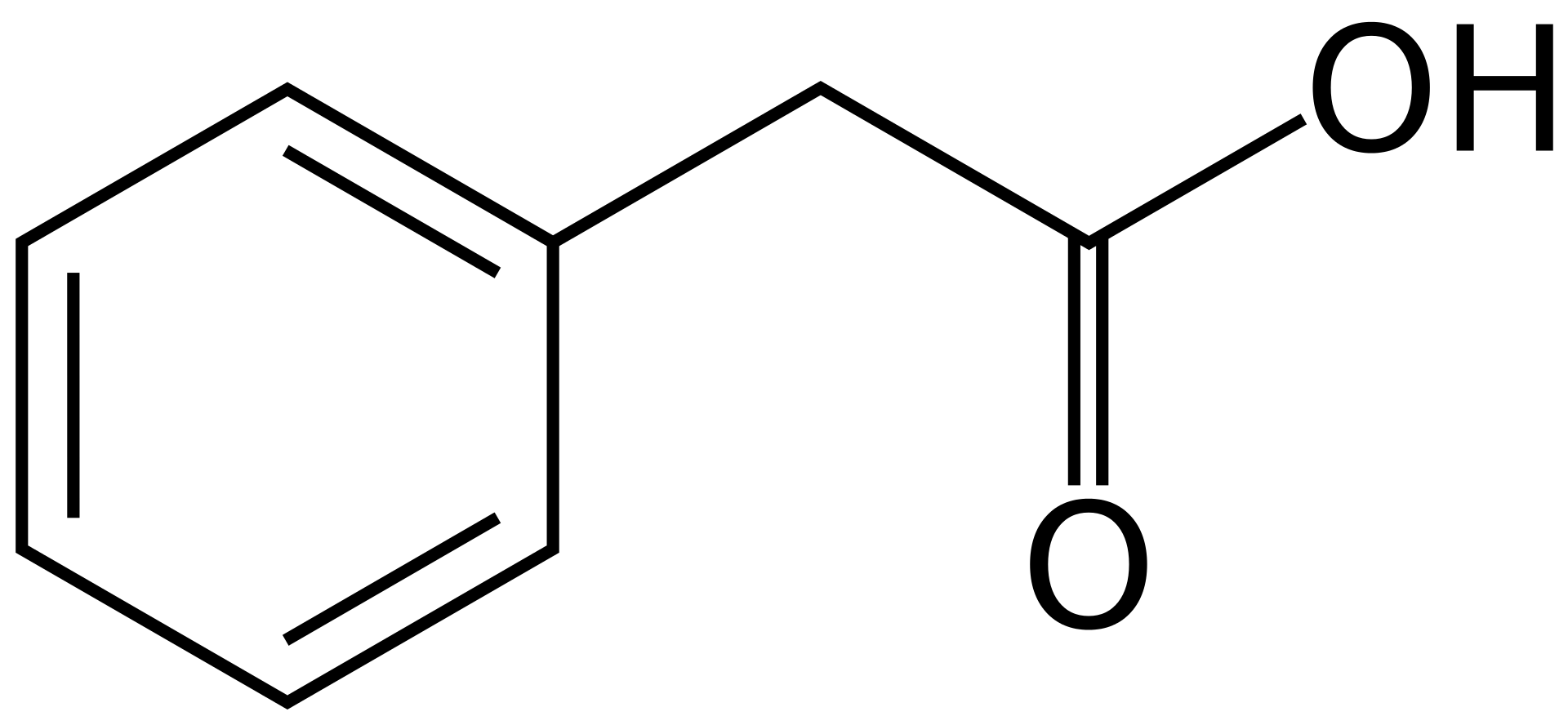
Appearance: white solid with a strong honey-like odor.
Moreover, in this topic you can find detailed benzyl chloride synthesis from benzene. In the end, I want to warn any chemists, who want to repeat this synthesis, that you have to use all safety equipment such as chemical glass, gloves, chemical coat and respiratory mask. Carry on experiments under pull out probe or under exhaust hood in good ventilated premises.
Appearance: white solid with a strong honey-like odor.
Boiling Point: 265.5 °C/760 mm Hg; 144.2-144.8 °C/12 mm Hg;
Melting Point: 76-77 °C;
Molecular Weight: 136.15 g/mol;
Density: 1.091 g/ml (20 °C).
Benzyl chloride from benzene
Equipment and glassware:
- 1 L three-necked flask;
- Laboratory grade thermometer (0 °C to 200 °C) with flask adapter;
- Reflux (double surface) condenser;
- Mechanical stirrer;
- Gas lead-in tube;
- Water bath;
- Heating plate;
- HCl laboratory generator;
- 1 L Separatory funnel;
- Distillation apparatus;
- Retort stand and clamp for securing apparatus;
- Water-jet aspirator;
- Laboratory scale (1 — 200 g is suitable);
- 100 ml x2 and 200 ml x2 Erlenmeyer flasks with cap;
- 500 ml Measuring cylinder.
Reagents:
- 200 g (227 ml) Benzene;
- 38 g of 40 % Formalin or 20 g of paraformaldehyde;
- 50 g Zinc chloride anhydrous (ZnCl2);
- 100 g Sodium bicarbonate (NaHCO3);
- 1 L Distilled water;
- 100 g Anhydrous calcium chloride (CaCl2)/magnesium sulfate (MgSO4).
Into a 1 litre three-necked flask, equipped with a reflux (double surface) condenser, a mechanical stirrer (preferably of the Hershberg type), and a gas lead-in tube extending to near the bottom of the flask, place 200 g (227 ml) of dry benzene, 20 g of paraformaldehyde (40% Formalin may also be used; the proportions are then 200 g of benzene, 38 g of 40% formalin and 50 g of pulverized zinc chloride) and 20 g of finely powdered anhydrous zinc chloride. Support the flask on a water bath so arranged that the level of the water in it is about the same height as the reaction. Heat the bath to 60 °C and pass in (through an intervening empty wash bottle) a rapid stream of hydrogen chloride until no more gas is absorbed (about 20 minutes): allow cooling. Transfer the reaction mixture to a separatory funnel, wash it successively with two 50 ml portions of cold water, two 50 ml portions of saturated sodium bicarbonate solution (It's essential to remove all the zinc salts in the washing process, otherwise the product largely resinifies during the distillation) and finally with 20 ml of water. Dry with anhydrous calcium chloride or magnesium sulfate, and distill under normal pressure from a Claisen flask with fractionating side arm until the temperature rises to 100-110 °C. After cooling somewhat, distill under reduced pressure and collect the benzyl chloride at 63-65 °C at 12 mmHg. The yield is 70 g. Some (about 4 g) p-xylylene dichloride, m.p. 100 °C, and a small amount of diphenylmethane are present in the residue in the flask.
Benzyl cyanide from benzyl chloride
Equipment and glassware:
- 5 L Round-bottom flask;
- Laboratory grade thermometer (0 °C to 200 °C) with flask adapter;
- Reflux condenser;
- Water bath;
- Heating plate;
- 2 L Drip funnel;
- 2 L Buchner flask and funnel Ø 25 cm (or Shott filter);
- Distillation apparatus;
- Retort stand and clamp for securing apparatus;
- Filter paper;
- Conventional funnel;
- Water-jet aspirator;
- Laboratory scale (1 - 1000 g is suitable);
- Magnetic stirrer;
- 1 L Measuring cylinder;
- 2 L x2; 500 ml x2 Beakers.
Reagents:
- 500 g (10 moles) Sodium cyanide (NaCN);
- 450 ml Distilled water;
- 1000 g (8 moles) Benzyl chloride;
- 1300 g Alcohol 95 % (EtOH);
- 775 ml Sulfuric acid aq. 50 % (H2SO4);
- 100 g Sodium bicarbonate (NaHCO3);
- 100 g Sodium chloride (NaCl).
In a 5000 ml round-bottomed flask, fitted with a stopper holding a reflux condenser and separatory funnel, are placed 500 g (10 moles) of powdered sodium cyanide (96-98 percent pure) and 450 ml of water. The mixture is warmed on a water bath in order to dissolve most of the sodium cyanide, and then 1000 g (8 moles) of benzyl chloride (bp 170-180 °C) mixed with 1000 g of 95% alcohol is run in through the drip funnel in the course of 30-45 minutes. The mixture is then heated under a reflux condenser on the steam bath for four hours, cooled and filtered with suction to remove most of the sodium chloride. It is well to wash the filtered salt with a small portion of alcohol in order to remove any benzyl cyanide which may have been mechanically held.
The flask is now fitted with a condenser, and as much alcohol as possible is distilled off on the steam bath. The residual liquid is cooled, filtered if necessary, and the layer of benzyl cyanide separated. This crude benzyl cyanide is now placed in a Claisen distilling flask and distilled under reduced pressure, the water and alcohol coming over first, and finally the cyanide (it is preferable to distill the last part of the solvents and the benzyl cyanide under vacuum). It is advantageous to use a fractionating column. The material is collected at 135-140 °C at 38 mm Hg (115-120 °C at 10 mm Hg). The yield is 740-830 g (80-90% of the theoretical amount). It is preferable to wash the final product in order to remove foul-smelling benzyl isocyanide, and to significantly lengthen the shelf-life of the product. The once-distilled benzyl cyanide is shaken vigorously for five minutes with an equal volume of warm (60 °C) 50% sulfuric acid, prepared by adding 275 ml of concentrated sulfuric acid to 500 ml of water. The benzyl cyanide is separated and washed with an equal volume of saturated sodium bicarbonate solution followed by an equal volume of half-saturated sodium chloride solution. It is then dried and distilled under reduced pressure. The loss in the washings is negligible.
The flask is now fitted with a condenser, and as much alcohol as possible is distilled off on the steam bath. The residual liquid is cooled, filtered if necessary, and the layer of benzyl cyanide separated. This crude benzyl cyanide is now placed in a Claisen distilling flask and distilled under reduced pressure, the water and alcohol coming over first, and finally the cyanide (it is preferable to distill the last part of the solvents and the benzyl cyanide under vacuum). It is advantageous to use a fractionating column. The material is collected at 135-140 °C at 38 mm Hg (115-120 °C at 10 mm Hg). The yield is 740-830 g (80-90% of the theoretical amount). It is preferable to wash the final product in order to remove foul-smelling benzyl isocyanide, and to significantly lengthen the shelf-life of the product. The once-distilled benzyl cyanide is shaken vigorously for five minutes with an equal volume of warm (60 °C) 50% sulfuric acid, prepared by adding 275 ml of concentrated sulfuric acid to 500 ml of water. The benzyl cyanide is separated and washed with an equal volume of saturated sodium bicarbonate solution followed by an equal volume of half-saturated sodium chloride solution. It is then dried and distilled under reduced pressure. The loss in the washings is negligible.
Phenylacetic acid from benzyl cyanide
Equipment and glassware:
- 5 L Three-necked round-bottom flask;
- Mechanical stirrer;
- Reflux condenser;
- Water bath;
- Heating plate;
- 2 l Buchner flask and funnel Ø 25 cm (or Shott filter);
- Conventional funnel;
- Filter paper;
- Distillation apparatus;
- Retort stand and clamp for securing apparatus;
- 1 L Measuring cylinder;
- 2 L x2; 500 ml x2 Beakers;
- Water-jet aspirator;
- Laboratory scale (1 - 1000 g is suitable);
- Laboratory grade thermometer (0 °C to 200 °C) with flask adapter;
- 2 l x2; 500 ml x2 Beakers.
Reagents:
- 3500 ml Distilled water;
- 840 ml Sulfuric acid conc. (H2SO4);
- 700 g (6 mol) Benzyl cyanide.
1150 ml of water, 840 ml of technical sulfuric acid and 700 g (6 mol) of benzyl cyanide are mixed in a 5-liter three-necked flask equipped with a mechanical stirrer. The flask is connected to a reflux condenser and heated for 3 hours with the stirrer running. After that, the contents of the flask are cooled to r.t., and then poured into 2 liters of cold water. In this case, the mass has to be stirred to prevent the formation of a solid cake. Obtained phenylacetic acid is filtered off on Buchner funnel. The reaction product is melted underwater and washed by decantation several times with hot water. After cooling the washings, a small amount of phenylacetic acid is obtained, which is filtered off and added to the main portion of the substance. Hot water from the last rinse is drained while the product is still molten. The liquid acid is poured into a 2 liter Claisen flask and distilled under vacuum. First, a small amount of water is distilled off and discarded. Then, about 20 ml of cut passes with a noticeable amount of benzyl cyanide. This fraction is used for the next synthesis. The next (third) fraction is distilled at a temperature of 176-189 °C (50 mm Hg); it is collected separately. After some standing time, the fraction solidifies.
Thus, the product obtained is a fairly pure phenylacetic acid with m.p. 76-76.5 °C; the yield is 630 g (77.5% of theory). Since the second fraction, which is used in the subsequent synthesis, contains a significant amount of phenylacetic acid, the overall product yield is at least 80% of theoretical. Phenylacetic acid has an unpleasant odor.
Thus, the product obtained is a fairly pure phenylacetic acid with m.p. 76-76.5 °C; the yield is 630 g (77.5% of theory). Since the second fraction, which is used in the subsequent synthesis, contains a significant amount of phenylacetic acid, the overall product yield is at least 80% of theoretical. Phenylacetic acid has an unpleasant odor.
Phenylacetic acid from benzyl chloride via Grignard reagent
Equipment and glassware:
- 250 ml Three-necked round-bottomed flask;
- Retort stand and clamp for securing apparatus;
- Mechanical stirrer with a joint;
- 500 ml Drip funnel with bypass;
- Reflux condenser;
- Calcium chloride tube;
- Heating plate;
- Water bath;
- Ice salt bath (-10 °C);
- Wolfe's Flask x2;
- Kipp's apparatus or solid carbon dioxide;
- 500 ml Separatory funnel
- Water-jet aspirator;
- Laboratory scale (1 - 100 g is suitable);
- Filter paper;
- 100 ml Measuring cylinder;
- 500 ml x2; 100 ml x2 Beakers;
- 1 L Buchner flask and funnel (or Shott filter).
Reagents:
- 2.4 g Magnesium shavings (Mg);
- 120 ml Diethyl ether;
- 12.7 g Benzyl chloride;
- 200 ml Sulfuric acid conc. (H2SO4);
- 10 g HCl (d 1.19) in 20 ml of distilled water and 100 ml 10% hydrochloric acid;
- 1 L Distilled water.
In a three-necked round-bottomed flask 250 ml, equipped with a mechanical stirrer with a joint, a drip funnel and a reflux condenser with a calcium chloride tube, place 2.4 g of magnesium shavings, add 30 ml of absolute ether and add 3-4 ml of a solution of 12.7 g of benzyl chloride in 50 ml of absolute ether. After the reaction begins, the ether becomes cloudy and boils (if necessary, to start the reaction, the flask is immersed in a bath with warm water), an ethereal solution of benzyl chloride is added dropwisely at such a rate that the ether boils calmly and evenly all the time. After the addition of all the benzyl chloride, the mixture is heated in a water bath for 1-1.5 h until the magnesium is completely dissolved.
Cool the flask strongly with a mixture of ice and salt (-10 °C), replace the drip funnel with a gas pipe and pass for 3-4 hours a not too strong current of dry carbon dioxide from a cylinder or Kipp's apparatus, drying the gas by passing through two Wolfe's Flasks with concentrated sulfuric acid (you can get a higher yield of phenylacetic acid by introducing solid carbon dioxide into the reaction).
Then replace the gas pipe with a drip funnel and, with strong cooling and stirring, add dropwisely a solution of 10 g HCl (d 1.19) in 20 ml of water. When the reaction mixture is divided into two transparent layers, the contents of the flask are transferred into a separatory funnel, shaken well and, after settling, the aqueous layer is separated. The latter is extracted twice with ether (20 ml portions). The combined ether solutions of phenylacetic acid are shaken again with a dilute sodium hydroxide solution, the alkaline solution is separated, acidified with 10% hydrochloric acid and cooled. The obtained phenylacetic acid is filtered under vacuum, washed with cold water, well wrung out and recrystallized from hot water. m.p. 75 °C, yield 8 g (60% theoretical).
Last edited by a moderator:
