William D.
Expert
- Joined
- Jul 19, 2021
- Messages
- 1,061
- Reaction score
- 1,343
- Points
- 113
1 žingsnis:
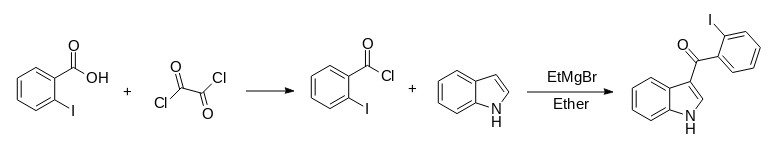
1. Į 1,00 g 2-jodobenzoinės rūgšties suspensiją 30 ml dichlormetano 0 *C temperatūroje lašiniu būdu įpilta 4,00 g oksalio chlorido.
2. Reakcijos mišinys buvo pašildytas iki kambario temperatūros ir maišytas 2 val.
3. Atvėsus, tirpiklis ir oksalichlorido perteklius buvo pašalinti vakuume ir gauta ruda liekana (0,90 g), kuri buvo panaudota kitame etape be tolesnio gryninimo.
4. 1,40 g indolo 5,0 ml eterio buvo lašiniu būdu įpilta į 0 *C temperatūroje maišytą 3,17 ml 2,5M etilmagnio bromido 2,5M eteryje tirpalą, atskiestą 1,1 ml eterio.
5. Tirpalas buvo maišomas 0,5 h kambario temperatūroje, po to lašiniu būdu buvo įpilta 0,90 g 2-jodobenzoilchlorido tirpalo 5 ml eterio.
6. Reakcijos mišinys buvo maišomas 1,5 h, gesinamas sočiu vandeniniu amonio chloridu ir maišomas tol, kol kietoji medžiaga suskilo į smulkią suspensiją.
7. Likutis buvo nuplautas vandeniu ir eteriu, po to suspenduotas 20 ml metanolio, į kurį pridėta 4 g natrio hidroksido ir 10 ml vandens.
8. Mišinys maišytas kambario temperatūroje 18 h, kietoji masė nufiltruota ir išplauta paeiliui metanolio, vandens ir eterio porcijomis.
9. Džiovinant vakuume 100 *C temperatūroje 1,06 g (70 %) 3-(2-jodobenzoil)indolo virto klampiu aliejumi, kuris buvo panaudotas kitame etape be tolesnio gryninimo.
2 žingsnis:
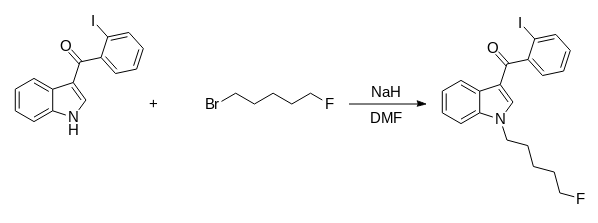
1. Į 1,06 g 3-(2-iodobenzoil)indolo tirpalo 10,0 ml N, N-dimetilformamido įpilta 0,5 g natrio hidrato.
2. Reakcijos mišinys buvo maišomas kambario temperatūroje ir lėtai įpilta 1,03 g 1-bromo-5-fluoropentano.
3. Tirpalas buvo maišomas 120 *C temperatūroje 1 val.
4. Atvėsęs reakcijos mišinys buvo praskiestas vandeniu ir ekstrahuotas trimis etilacetato porcijomis.
5. Ekstraktai buvo nuplauti sūrymu ir išdžiovinti, o tirpiklis pašalintas vakuume.
6. Gauta 0,60 g (30 %) 1-(heptil-7-karboksilato)-3-(2-jodobenzoil)indolo kaip geltonos spalvos kietoji medžiaga.
1. Į 1,00 g 2-jodobenzoinės rūgšties suspensiją 30 ml dichlormetano 0 *C temperatūroje lašiniu būdu įpilta 4,00 g oksalio chlorido.
2. Reakcijos mišinys buvo pašildytas iki kambario temperatūros ir maišytas 2 val.
3. Atvėsus, tirpiklis ir oksalichlorido perteklius buvo pašalinti vakuume ir gauta ruda liekana (0,90 g), kuri buvo panaudota kitame etape be tolesnio gryninimo.
4. 1,40 g indolo 5,0 ml eterio buvo lašiniu būdu įpilta į 0 *C temperatūroje maišytą 3,17 ml 2,5M etilmagnio bromido 2,5M eteryje tirpalą, atskiestą 1,1 ml eterio.
5. Tirpalas buvo maišomas 0,5 h kambario temperatūroje, po to lašiniu būdu buvo įpilta 0,90 g 2-jodobenzoilchlorido tirpalo 5 ml eterio.
6. Reakcijos mišinys buvo maišomas 1,5 h, gesinamas sočiu vandeniniu amonio chloridu ir maišomas tol, kol kietoji medžiaga suskilo į smulkią suspensiją.
7. Likutis buvo nuplautas vandeniu ir eteriu, po to suspenduotas 20 ml metanolio, į kurį pridėta 4 g natrio hidroksido ir 10 ml vandens.
8. Mišinys maišytas kambario temperatūroje 18 h, kietoji masė nufiltruota ir išplauta paeiliui metanolio, vandens ir eterio porcijomis.
9. Džiovinant vakuume 100 *C temperatūroje 1,06 g (70 %) 3-(2-jodobenzoil)indolo virto klampiu aliejumi, kuris buvo panaudotas kitame etape be tolesnio gryninimo.
2 žingsnis:
1. Į 1,06 g 3-(2-iodobenzoil)indolo tirpalo 10,0 ml N, N-dimetilformamido įpilta 0,5 g natrio hidrato.
2. Reakcijos mišinys buvo maišomas kambario temperatūroje ir lėtai įpilta 1,03 g 1-bromo-5-fluoropentano.
3. Tirpalas buvo maišomas 120 *C temperatūroje 1 val.
4. Atvėsęs reakcijos mišinys buvo praskiestas vandeniu ir ekstrahuotas trimis etilacetato porcijomis.
5. Ekstraktai buvo nuplauti sūrymu ir išdžiovinti, o tirpiklis pašalintas vakuume.
6. Gauta 0,60 g (30 %) 1-(heptil-7-karboksilato)-3-(2-jodobenzoil)indolo kaip geltonos spalvos kietoji medžiaga.
Last edited by a moderator:
