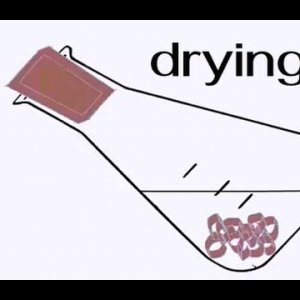
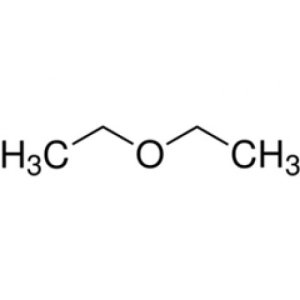
Drying of Organic Liquids
https://bbgate.com/threads/decantation-gravity-filtration-and-liquid-transferring.566/
https://bbgate.com/threads/decantation-gravity-filtration-and-liquid-transferring.566/
About Us
Our team brings together the best specialists from different fields.
We are ready to share our experience, discuss difficult issues and find new solutions.