G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,773
- Solutions
- 3
- Reaction score
- 3,002
- Points
- 113
- Deals
- 1
What is pH?
pH Is a measure of hydrogen ion concentration, a measure of the acidity or alkalinity of a solution. The pH scale usually ranges from 0 to 14. Aqueous solutions at 25°C with a pH less than 7 are acidic, while those with a pH greater than 7 are basic or alkaline. A pH level of 7.0 at 25°C is defined as "neutral" because the concentration of H3O+ equals the concentration of OH− in pure water. Very strong acids might have a negative pH, while very strong bases might have a pH greater than 14.
pH Is a measure of hydrogen ion concentration, a measure of the acidity or alkalinity of a solution. The pH scale usually ranges from 0 to 14. Aqueous solutions at 25°C with a pH less than 7 are acidic, while those with a pH greater than 7 are basic or alkaline. A pH level of 7.0 at 25°C is defined as "neutral" because the concentration of H3O+ equals the concentration of OH− in pure water. Very strong acids might have a negative pH, while very strong bases might have a pH greater than 14.
pH Equation
The equation for calculating pH was proposed in 1909 by Danish biochemist Søren Peter Lauritz Sørensen:pH = -log[H+]
Where log is the base-10 logarithm and [H+] stands for the hydrogen ion concentration in units of moles per liter solution. The term "pH" comes from the German word "potenz," which means "power," combined with H, the element symbol for hydrogen, so pH is an abbreviation for "power of hydrogen."
Not all liquids have a pH value
pH only has meaning in an aqueous solution (in water). Many chemicals, including liquids, do not have pH values. If there's no water, there's no pH. For example, there is no pH value for vegetable oil, gasoline, or pure alcohol.How pH Is measured
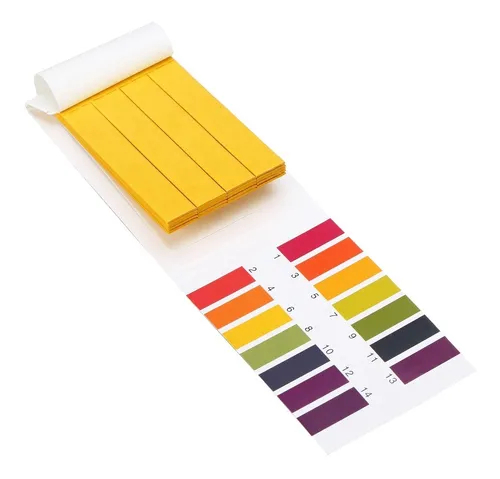
Rough pH measurements can be made using litmus paper or another type of pH paper known to change colors around a certain pH value. Most indicators and pH papers are useful only to tell whether a substance is an acid or a base, or to identify pH within a narrow range. A universal indicator is a mixture of indicator solutions intended to provide a color change over a pH range of 2 to 10.More accurate measurements are made using primary standards to calibrate a glass electrode and pH meter. The electrode works by measuring the potential difference between a hydrogen electrode and a standard electrode. An example of a standard electrode is silver chloride.
Get to know the difference between pH paper and litmus paper. To obtain an accurate reading of a solution, you can use pH paper. This is not to be confused with the common litmus paper. Both can be used to test for acids and bases, but they differ in important ways. pH paper will tell you the actual pH value of the water, but litmus strips typically only indicate whether the water is basic or acidic.
pH Strips contain a series of indicator bars that will all change color after exposure to a solution. The strength of the acids and bases on each bar differs. After they change, the color pattern of the bars can be matched to the examples that come with the kit.
Litmus papers are strips of paper that contain an acid or a base (alkaline). The most common of these are red (which contains an acid that reacts with bases) and blue (which contains a base that reacts with acids). The red strips turn blue if the substance is alkaline, and the blue strips turn red if they contact an acid. Litmus papers can be used to provide a quick and easy test, but the cheapest of them do not always provide accurate readings on the strength of the solution.
Get to know the difference between pH paper and litmus paper. To obtain an accurate reading of a solution, you can use pH paper. This is not to be confused with the common litmus paper. Both can be used to test for acids and bases, but they differ in important ways. pH paper will tell you the actual pH value of the water, but litmus strips typically only indicate whether the water is basic or acidic.
pH Strips contain a series of indicator bars that will all change color after exposure to a solution. The strength of the acids and bases on each bar differs. After they change, the color pattern of the bars can be matched to the examples that come with the kit.
Litmus papers are strips of paper that contain an acid or a base (alkaline). The most common of these are red (which contains an acid that reacts with bases) and blue (which contains a base that reacts with acids). The red strips turn blue if the substance is alkaline, and the blue strips turn red if they contact an acid. Litmus papers can be used to provide a quick and easy test, but the cheapest of them do not always provide accurate readings on the strength of the solution.
Spet-by-step manual
pH Indicator Strips
pH indicator strips are a qualitative method for determining the pH of a solution. A strip of paper containing a pH indicator is dipped into a solution of unknown pH. The part of the strip dipped into the solution will change color depending on the acidity of the solution.
pH indicator strips are often composed of a mix of indicators so that they can be used as universal indicators. They turn red in very acidic solutions (pH < 3), green in neutral solutions (pH = 7), and violet in very basic solutions (pH > 11).
pH indicator strips are often composed of a mix of indicators so that they can be used as universal indicators. They turn red in very acidic solutions (pH < 3), green in neutral solutions (pH = 7), and violet in very basic solutions (pH > 11).
pH strips determine the pH of a solution by displaying a specific color. They contain pH indicators that change color depending on the pH.
pH indicator strips are one way to measure the pH of a solution between pH 1 and 14. They contain a universal indicator that changes color depending on the pH of the solution they are dipped into. At lower acidic pH values between 1 and 4, the strip color ranges from red to orange. Around the neutral pH between values of 5 and 9, the color ranges from yellow to green. At higher basic pH values between 10 and 14, the strip color ranges from dark greenish-blue to purple.
Let’s look at an example of how to use a pH indicator strip. You have a solution, and you are interested in finding its pH. To determine the pH, you would:
pH indicator strips are one way to measure the pH of a solution between pH 1 and 14. They contain a universal indicator that changes color depending on the pH of the solution they are dipped into. At lower acidic pH values between 1 and 4, the strip color ranges from red to orange. Around the neutral pH between values of 5 and 9, the color ranges from yellow to green. At higher basic pH values between 10 and 14, the strip color ranges from dark greenish-blue to purple.
Let’s look at an example of how to use a pH indicator strip. You have a solution, and you are interested in finding its pH. To determine the pH, you would:
- Dip the pH indicator strip into the solution.
- Wait for the color of the strip to stop changing.
- Remove the strip from the solution and compare the color of the strip against a key which contains pH values and their respective colors.
To measure the pH of a solution, pH indicator strips are first dipped into the solution until the color of the strip no longer changes. Then, the strip color is compared with a color wheel of standard pH values ranging from pH 1 to pH 14. For example, if the color of the strip is orange, the pH of the solution is around 4.
In case If you want to measure pH in big flask or have limited volume of a solution, you can take one drop with helps of glass rod. Just dip the end of the rod into solution and then put the drop from the end of the glass rod on a pH indicatory paper.
Using a pH meter
Calibrate the probe and meter following the manufacturer specifications. You may need to calibrate the meter by testing it in a substance with a known pH rating. You can then adjust the meter accordingly. If you will be testing water away from a lab, you may want to perform this calibration several hours before you take the meter to the field.
1. Rinse the probe with double deionized water before using it. Dry it off with a clean tissue.
1. Rinse the probe with double deionized water before using it. Dry it off with a clean tissue.
2. Collect a sample of the water or test solution in a clean container. The sample must be deep enough to cover the tip of the electrode. Let the sample sit for a moment, so the temperature can stabilize, then measure the temperature of the sample using a thermometer.
3. Adjust the meter to match the sample temperature. The probe's sensitivity is affected by the temperature of the water, and so the reading of the meter cannot be accurate if you do not input the temperature data. The pH of the water will also be affected by the water’s temperature—pure water has a lower pH at higher temperatures and a higher pH at lower temperatures.
3. Adjust the meter to match the sample temperature. The probe's sensitivity is affected by the temperature of the water, and so the reading of the meter cannot be accurate if you do not input the temperature data. The pH of the water will also be affected by the water’s temperature—pure water has a lower pH at higher temperatures and a higher pH at lower temperatures.
4. Put the probe into the sample. Wait for the meter to come to equilibrium. The meter has reached equilibrium when the measurement becomes steady.
5. Read the pH measurement of the sample. Your pH meter should provide a reading on the scale of 0-14. If the water is pure, it should read close to 7. Record your findings.
- A pH reading lower than 7 indicates that the water is acidic, while a reading higher than 7 indicates that the water is basic.
Last edited by a moderator:
