You should upgrade or use an alternative browser.
Psilocybin & Psilocin: Comprehensive Synthesis Guide
Psilocybin & Psilocin: Comprehensive Synthesis Guide
Introduction
Psilocybin and psilocin are naturally occurring psychedelic compounds found in certain species of mushrooms. These compounds have been used for centuries in various cultural and spiritual practices, and in recent years, they have gained significant attention in scientific research for their potential therapeutic applications.
Synthesis of Pcilocin
Pcilocin Synthesis Reagents
- 1H-indol-4-yl acetate 50.1 g (285 mmol, 1 equiv);
- Diethyl ether Et2O anhydrous 760 ml;
- Oxalyl chloride 37.1 ml (428 mmol,1.5 equiv);
- Heptane 2425 ml;
- Tetrahydrofuran THF 1600 ml;
- Dimethylamine solution 2.0 M in THF 175 ml;
- Pyridine 46 ml;
- Deionized H2O 1000 ml;
- Ethyl acetate EtOAc 6255 ml;
- 2-Me-THF 1020 ml;
- Lithium alumohydride (LiAlH4) 2.3 M solution in 2-Me-THF (140 ml, 322 mmol);
- Sodium hydroxide (NaOH aq. 1 M);
- 7% Ammonia (NH3) in MeOH solution 10% in CH2Cl2 (200 ml);
3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate Synthesis
1. A round-bottomed flask, equipped with an overhead stirrer, temperature controller, and dropping funnel, 1H-indol-4-yl acetate (50.1 g, 285 mmol, 1 equiv) and dry Et2O (700 ml) are added .
2. The suspension is stirred for 10 min and then cooled to 0 ℃ in an ice-water bath for 30 min.
3. The dropping funnel is charged with a solution of oxalyl chloride (37.1 ml, 428 mmol,1.5 equiv) in Et2O (60 ml).
4. The oxalyl chloride solution is added dropwise at a rate, sufficient to maintain the temperature ≤ 5 ℃, in order to minimize a dimer and other possible by-product formation.
5. As the addition progressed, a yellow slurry is formed and when the addition is completed, the mixture is stirred for 4 h.
6. After this, heptane (400 ml) is added and the mixture stirred for 30 min at 0 ℃.
7. The obtained yellow solid is quickly filtered and rinsed successively with heptane (2x300 ml), which is quickly dissolved in THF (500 ml) and cooled to 0 ℃.
8. A 2.0 M solution of dimethylamine in THF (175 ml) is added dropwise at a rate sufficient to maintain temperature below 5 ℃ in order to minimize side reactions.
9. After the addition is complete, pyridine (46 ml) in of THF (100 ml) is added dropwise and the mixture is stirred well for 60 min.
10. Heptane (600 ml) is added, and the flask content is suction filtered via Büchner funnel.
11. The filtered residue is transferred into a roundbottomed flask and deionized H2O (1000 ml) is added, stirred for 30 min, and filtered via Büchner funnel.
12. The off-white solid is triturated sequentially for 40 min in EtOAc (600 ml) and heptane (400 ml).
13. The slurry is filtered via Büchner funnel and the solid is dried in an oven at 40 ℃ overnight to afford 3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate as a light-yellow solid; yield is 66.1 g (81%).
Psilocin Synthesis (3-[2-(Dimethylamino)ethyl]-1H-indol-4-ol)
Procedure A:
1. A round-bottomed flask, equipped with an overhead stirrer, temperature controller, and a dropping funnel, is added 3-[2-(dimethylamino)-2-oxoacetyl]-1H-indol-4-yl acetate (31.5 g, 115 mmol) and 2-Me-THF (1000 ml).
2. The flask is immersed into an ice-bath at 0 ℃ and a solution of 2.3 M LiAlH4 in 2-Me-THF (140 ml, 322 mmol) is added through the dropping funnel.
3. The dropping funnel is rinsed with an additional 2-Me-THF (20 ml). The LiAlH4 solution is added dropwise at a rate to maintain a temperature below 20 ℃.
4. After the addition, the ice-water bath is removed and the mixture stirred for 30 min.
5. The light-yellow solution is heated to reflux (80 ℃) with a heating mantle and became ivory-colored after 3 h.
6. An accumulation of yellow solids is observed on the sides of the round-bottomed flask.
7. The heating mantle is removed, and the flask is allowed to cool down to 50 ℃.
8. The flask is chilled again to 20 ℃.
9. The reaction is quenched by sequential addition of 3 drops of aq. 1 M NaOH and 3 drops of deionized H2O.
10. The mixture is diluted with THF (500 ml) and stirred for 20 min.
11. The mixture is filtered via Büchner funnel and the filtrate is kept under N2.
12. The filter cake is quickly reslurried with 200 ml 10% solution of (7% ammonia in MeOH) in CH2Cl2 and THF (500 ml).
13. The filtrates are then combined and concentrated to give a green solid.
14. The solid is triturated with 1:1 EtOAc/heptane (50 ml), then filtered via Büchner funnel.
15. The dark green solid is dried in an oven at 40 ℃ overnight to provide dry psilocin as a dark green solid; Psilocin yield is 20.7 g (91%); mp 167–169 ℃.
Procedure B:
1. The reduction step is carried out using essentially the same protocol described in Procedure A with 3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate (40.21 g, 135.2 mmol) and 2.3 M LiAlH4 in 2-Me-THF (188.1 ml, 432.5 mmol).
2. The reaction is quenched by dropwise addition of THF/H2O (27:100, 50 ml) at a rate that kept the temperature below 30 ℃.
3. Anhydrous Na2SO4 (100 g) is added, followed by silica gel (50 g) and DCM (400 ml).
4. The mixture is stirred for 10 min and is filtered via Büchner funnel.
5. The filter cake is washed with DCM/CH3OH mixture (9:1, 1500 ml).
6. The filtrates are then combined and concentrated to give a light green solid.
7. The solid is triturated with 1:1 EtOAc/heptane (50 ml), then filtered via Büchner funnel.
8. The off-white solid is dried in an oven at 40 ℃ overnight to provide dry psilocin as an off-white solid; Psilocin yield is 21.6 g (77%).
Total Synthesis of Psilocybin
Psilocybin Synthesis Reagents
- Psilocin (10.3 g, 60.2 mmol);
- Tetrahydrofuran THF (500 ml);
- n-Butyllithium (BuLi) 2.5M in hexanes (28.9 ml, 72.3 mmol);
- Tetrabenzyl pyrophosphate (35.7 g, 66.2 mmol);
- Ethyl acetate EtOAc 1400 ml;
- Dichloromethane DCM 500ml;
- Methanol CH3OH 1700 ml;
- Pd/C 10% 1.1 g;
- i-PrOH 200 ml;
- Deionized H2O (~50 ml);
- Acetone;
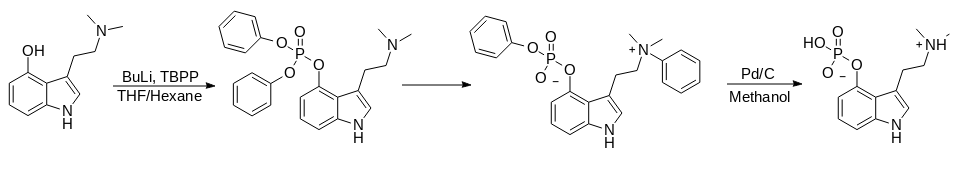 Benzyl {3-[2-(Benzyldimethylammonio)ethyl]-1H-indol-4-yl} Phosphate Synthesis
Benzyl {3-[2-(Benzyldimethylammonio)ethyl]-1H-indol-4-yl} Phosphate Synthesis
1. Into a round-bottomed flask, equipped with an overhead stirrer, temperature controller, and dropping funnel, psilocin (10.3 g, 60.2 mmol) and anhydrous THF (500 ml) are added.
2. The mixture is stirred for 15 min and the flask is immersed in a solid CO2/acetone cooling bath at –78 ℃.
3. When the internal temperature of the reaction reached –67 ℃, a solution of 2.5M BuLi in hexanes (28.9 ml, 72.3 mmol) is added dropwise over a period of a few min and maintained the internal temperature reading below –60 ℃.
4. After stirring the olive-green colored reaction mixture for 10 min, tetrabenzyl pyrophosphate (35.7 g, 66.2 mmol) is added in one portion and the mixture is stirred well.
5. After 1.5h, the solid CO2/acetone cooing bath is removed and the temperature is allowed to slowly rise to –25 ℃ over 2 h.
6. Amino bound silica gel (30 g) is added in one portion and the reaction is diluted with EtOAc (600 ml).
7. The dark mixture is filtered through a pad of Celite and washed with EtOAc (400 ml).
8. The filter cake is reslurried for 10 min with EtOAc (400 ml) and again filtered.
9. The combined filtrates are concentrated and transferred into a 500 ml single-necked round-bottomed flask.
10. The gray oil is redissolved in DCM (100ml) and heated with a heat gun to boiling for 5 min.
11. The flask is allowed to reach rt and then held at 4 ℃ overnight.
12. The crude grayish-colored benzyl {3-[2-(Benzyldimethylammonio)ethyl]-1H-indol-4-yl} phosphate zwitterion precipitate is filtered via Büchner funnel, then triturated with DCM (4x100 ml).
13. The zwitterion precipitate is transferred into a 250 ml single-necked round-bottomed flask and thoroughly dried in the vacuum oven at 40 ℃ overnight to provide a light-purple solid; Benzyl {3-[2-(Benzyldimethylammonio)ethyl]-1H-indol-4-yl} Phosphate yield is 19.2 g (63%).
Psilocybin Synthesis From Benzyl {3-[2-(Benzyldimethylammonio)ethyl]-1H-indol-4-yl} Phosphate
1. Into a 2000 ml round-bottomed flask is added benzyl {3-[2-(benzyldimethylammonio)ethyl]-1H-indol-4-yl} phosphate (16.9 g, 35.6 mmol) followed by CH3OH (1200 ml).
2. The mixture is degassed and refilled with N2.
3. 10% Pd/C (1.1 g) is added, and the mixture is degassed and refilled with H2 balloon at 1 atm.
4. The reaction mixture is stirred overnight at room temperature.
5. The flask is degassed, refilled with N2 and the suspension is filtered through a pad of Celite via Büchner funnel.
6. The filter pad is washed with CH3OH (500 ml) and the purple colored filtrate is concentrated and dried overnight under vacuum to give 10.7 g of crude Psilocybin (106%).
7. The crude solid is suspended in i-PrOH (200 ml) and boiled for 30 min, then filtered hot (50 to 60 ℃).
8. The collected solid is washed with acetone to give a pale purple colored solid.
9. The purple solid is then suspended in 25% CH3OH/i-PrOH and boiled for 30 min and filtered hot, washing with 25% CH3OH/i-PrOH to give a light purple solid.
10. Finally, the solid is recrystallized from 30% H2O in acetone and filtered to give light blue needles.
11. Further recrystallization from 30% acetone/water gave colorless needles.
12. A final recrystallization from deionized H2O (~50 ml) gave a white solid, which is dried in an oven at 60 ℃ for two days to provide as a white solid; Psilocybin yield is 4.9 g (49%).
-

Psilocybin and psilocin extraction from mushrooms
Introduction A simple aqueous extraction method for the isolation and identification of psilocin from Psilocybe Cubensis mushrooms is reported. This method employs a dephosphorylation of the phosphate ester to psilocin, which facilitates a greater product yield and simplifies identification...- G.Patton
- Thread
- extraction mushrooms pcilocybin psilocin psilocybin psilocybin and psilocin extraction from mushrooms
- Replies: 29
- Forum: Tryptamines
-

Psilocybin | General pharmacology
Psilocybin (3-[2-(Dimethylamino)ethyl]-1H-indol-4-yl dihydrogen phosphate) is an alkaloid of a tryptamine family, phosphorylated psilocin derivative, which has psychedelic properties. Out of all biological sources of psilocybin, mushroom species of genera Psilocybe, Panaeolus, Stropharia...- Brain
- Thread
- pcilocybin
- Replies: 0
- Forum: Tryptamines
-

Synthesis of Pcilocybin
Reaction scheme: Benzyl {3-[2-(Benzyldimethylammonio)ethyl]-1H-indol-4-yl} Phosphate. 1. Into a round-bottomed flask was equipped with an overhead stirrer, temperature controller, and dropping funnel was added psilocin (10.3 g, 60.2 mmol) and anhyd THF (500 ml). 2. The mixture was stirred for...- WillD
- Thread
- pcilocybin synthesis of pcilocybin
- Replies: 0
- Forum: Tryptamines
-

Synthesis of Pcilocin
3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate. 1. Into a round-bottomed flask was equipped with an overhead stirrer, temperature controller, and dropping funnel was added 1H-indol-4-yl acetate (50.1 g, 285 mmol, 1 equiv) and anhyd Et2O (700 ml). 2. The suspension was stirred for 10...- WillD
- Thread
- pcilocin synthesis pcilocybin synthesis of pcilocin
- Replies: 3
- Forum: Tryptamines
About Us
Our team brings together the best specialists from different fields.
We are ready to share our experience, discuss difficult issues and find new solutions.
Mirror Links
Help Pages
Follow us
Popular Tags
-
Free product samples
Testing products from new vendors and manufacturers.
Get free samples for testing now!
-
Always stay in touch with BB forum. Element/Matrix.
Connect notifications to always stay in touch with the forum!
Connect
-
The BB Forum team is looking for cooperation:
- Traffic arbitrage specialists
- Spammers
- Advertising agencies
- Bloggers/Vloggers
- TOR sites directories
- Creative people who can create viral content
- Administrators of Telegram Channels and Groups
We will pay more for your traffic than our competitors! $0.1 per visitor!!!If you are interested in, write to the administrator.
