MDMA (3,4-Methylenedioxy-N-methylamphetamine) (molly, mandy, emma, MD, ecstasy, E, X, XTC, rolls, beans) – is a psychoactive substance which belongs to the phenylethylamine class. The use of MDMA is associated with stimulatory effects, euphoria, contentment and so on. MDMA has a complex pharmacological profile, mainly consisting of its effects as an inhibitor of release and reuptake of monoamines and its additional effects involving limitation of the synthesis and degradation of neurotransmitters. It has a significant effect on serotonin, unlike amphetamine

and metamphetamine which mainly increase the number of catecholamines. This substance was first synthesized in 1912 by Anton Köllisch, which worked on finding an effective hemostatic agent (a precursor of a hemostatic drug methylhydrastinine as phenylisopropylamine derivative of safrol, the latter being an aroma oil found i sassafras, nutmeg and other plants) under the auspices of the company Merck KGaA (of Darmstadt, Germany). In 1914, the substance was patented. In 1927 Max Oberlin found that MDMA effects on vascular smooth muscle tissue were similar to those of adrenaline and ephedrine, he also claimed that this substance had hemostatic and uterotonic properties. However, later the research was discontinued due to an increase in price of safrylmethylamine. Pharmacological trials were carried out by Merck in 1952 and 1959. Toxic effects were secretly studied on laboratory animals by the USA Army in the University of Michigan in 1953-1954. In 1960 two polish chemists (Biniecki and Krajewski) published a paper, describing the synthesis process of MDMA, which was eventually published in Chemical Abstracts in 1961. There were not any official human trials on MDMA until 1970. The American chemist Alexander T. Shulgin, who had a great interest in psychoactive drugs (having, for example, synthesised the closely related 3,4-methylene-dioxyethylamphetamine or MDE in 1967), mentioned once that he had first synthesised MDMA in 1965, but this date has not been reliably verified. Information about the appearance of MDMA in the USA as a recreational drug is vague, however, M. M. Kirsch, a writer from Los Angeles, stated in his book “Designer Drugs”, that “a number of black-market chemists had synthesized it during the 1960s but found LSD and MDA more profitable”. In publication as of 1997 "The early history of MDMA" Shulgin told about an occasional exchange of opinions which he had had with a chemist, who owned a chemical company in Los Angeles. The chemist asked Shulgin to help him synthesize DOB and MDMA. During an August 1970 conference of the American Society for Pharmacology and Experimental Therapeutics held at Stanford University, Shulgin happened to meet a young “pharmacologist/psychologist” with the same name as the chemist’s customer from the Midwest. This person had come to San Francisco to study street drugs with the Haight Ashbury Free Medical Clinic. A while later, Shulgin was informed that the young pharmacologist/psychologist had gone back to the Midwest. In August 1970, the Chicago Police Department seized the first MDMA sample in the U.S. Data from the analysis were first announced at a meeting of crime laboratory chemists. The author presented findings on “a new series of amphetamines,” among them DOM, TMA, MDA, and the then virtually unknown MDMA.DEA officials reported that this and other “...laboratories seized were believed to be making a controlled substance (MDA),” but were found to be producing MDMA. Consequently, “investigations were not continued due to the noncontrolled status of MDMA”. In 1974, DEA labs analyzed five MDMA street samples from Champaign, Illinois, and Aspen, Colorado.Keith Bailey and his colleagues at the Research Laboratories of the Health Protection Branch in Ottawa, Canada, submitted a scientific manuscript in August 1974 in which they identified five N-methylated analogues of hallucinogenic amphetamines and reported that MDMA “has been encountered on the illicit market” in Canada. A laboratory producing MDMA was raided in Ontario, Canada, in early 1976, and consequently MDMA was scheduled in Canada on 11 June 1976.
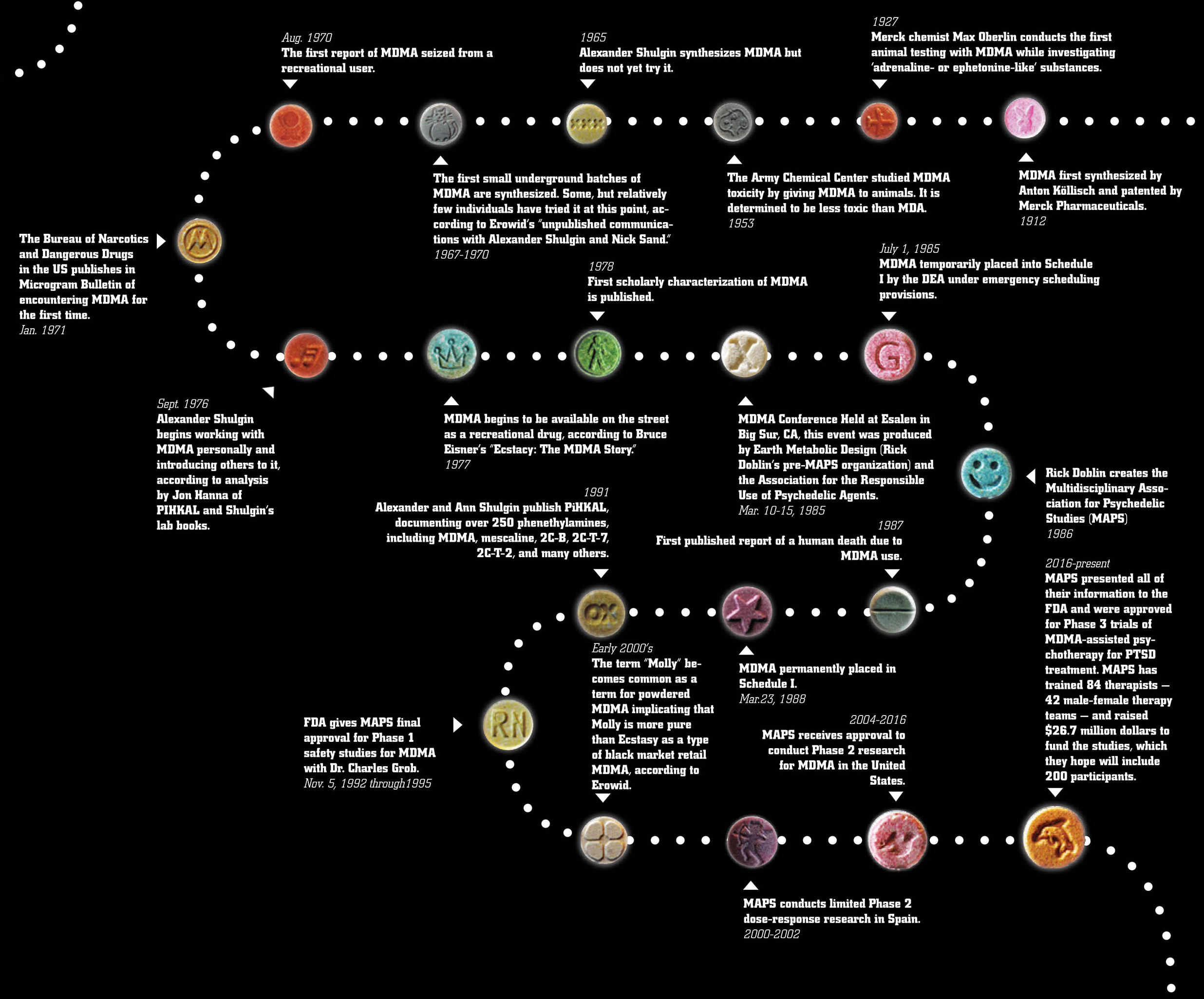
Around 1975, Alexander Shulgin, also based on the West Coast, again became involved with MDMA. Shulgin met a young student who was interested in drugs, especially in “some N-methylated compounds” (as is MDMA). The student had found in self experiments that MDMA had a significant “amphetamine-like component”. In his laboratory notebook, Shulgin referred to this student a
s “Marty” ("~1975: Marty—reports considerable amphetamine-like content". Probably in the same year, Shulgin met another person who had used MDMA. In a box on the right side of page 186 of his laboratory notebook (it is unclear when Shulgin added it), the trials of a certain “Flip” with “N-methyls,” especially with “N-methylated MDA” (i.e., MDMA), are listed. “Flip” had taken 15, 30, 45, 60, 75, 100, and 150 mg of MDMA. Doses of up to 60 mg had “no effect,” 75 mg made him “fuzzy,” 100 mg and 150 mg made him “active”. Given the circumstances, it is prob- able that “Flip” is a colleague from the University of San Francisco, whom Shulgin identified as someone who had synthesized some N-methylated phenethylamines in the 1970s. It was active from 1972 to 1983 and analyzed more than 20,000 street drug samples. In 1981, an early distributor of MDMA was quoted in the underground magazine WET: “We first started distributing Ecstasy five years ago...”. This would make 1976 the first year of its distribution as a recreational drug. As far as can be reconstructed from the literature, the name “ecstasy” was coined by the former student of theology and later proselytizer of MDMA Michael Clegg in 1981. In mid-1977, Alexander Shulgin handed over some MDMA to one of his long-term acquaintances, the psychotherapist Leo Zeff, who by the late 1960s had become the “secret chief” of a circle of underground therapists using psychedelics in psychotherapy. Zeff’s response to MDMA was enthusiastic, and he postponed his retirement plans to disseminate knowledge about MDMA among hundreds of fellow psychotherapists. The well-known drug guru Timothy Leary took his first MDMA trips in 1978 on the East Coast. Apparently, he did not immediately go public with his enthusiastic response to this new “empathy-generating drug.” His description was published much later, but Leary served to widen distribution of MDMA through his personal connections. Shulgin and Nichols’ presentation at a 1976 NIDA conference was published in the conference proceedings in 1978. Additionally, Shulgin made other scientific presentations and publications in 1978, and these contributed to the widening knowledge about MDMA’s effects. Shulgin’s own self-trials with MDMA began in September 1976, and he presented psychopharmacological findings on MDMA at a NIDA conference in December 1976. In mid-1977, he handed over some MDMA to psychotherapist Leo Zeff, later a proselytizer of MDMA in psychotherapy on a national scale. In 1978, Shulgin spoke about or published on MDMA on three occasions. Yet, in the broad picture, it looks more as if “MDMA had come across Shulgin”, than Shulgin had come across MDMA. At the beginning of the 1980s, the use of MDMA successively spread further throughout the U.S. With an estimated 10,000 pills distributed per year until the end of the 1970s, its use escalated to 30,000 pills per month estimated for 1983. Mainly due to its escalating use in some larger Texas cities beginning in 1983, U.S. senators intervened by urging the DEA to schedule it for endangering of the young. The DEA initiated procedures necessary for its ban and MDMA was scheduled on 1 July 1985.
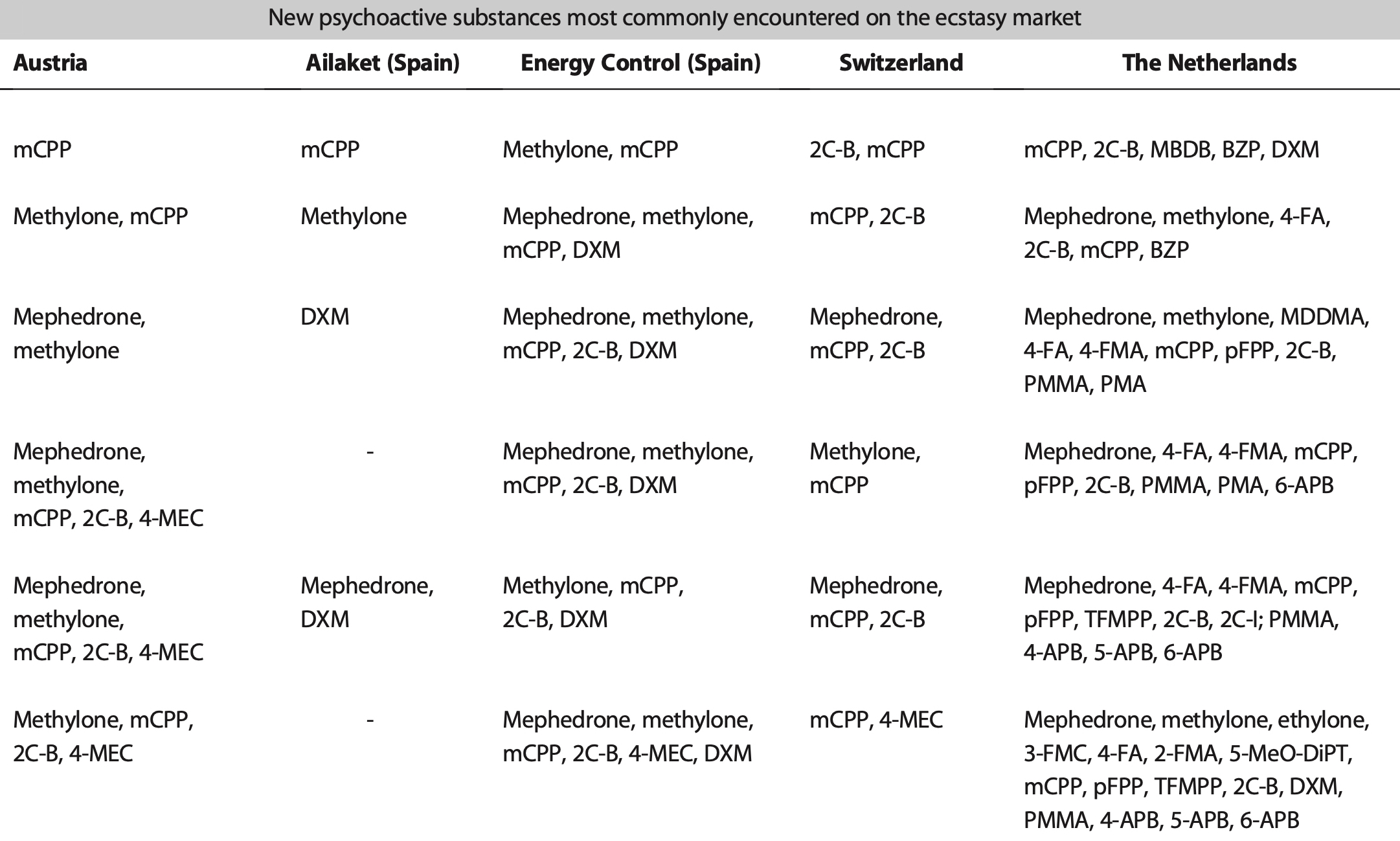
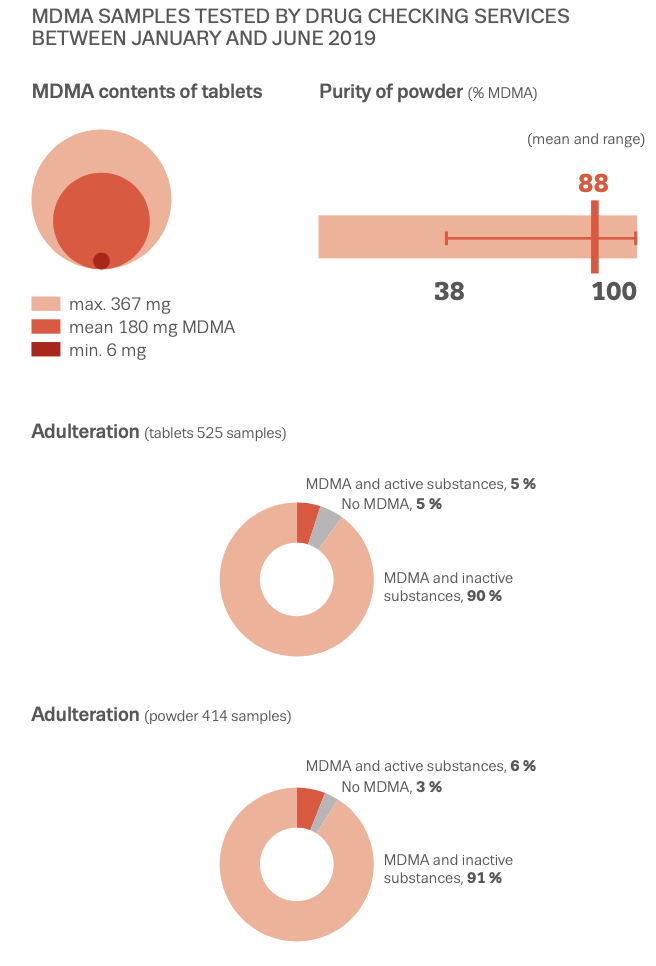
While misselling of other drugs as MDMA pills has tailed off in recent years (although a problem that could return as 2021 data from the loop suggests), what might have been a positive development in some respects (greater certainty for consumers of the substance they are purchasing and a reduction in more risky adulterants and misselling) has been countered by the growing risks from high potency MDMA pills and powder. The EMCDDA Trendspotter notes that ‘over half (53%) of all ecstasy tablets tested in 2015 contained over 140 milligrams of MDMA, compared to just 3% in 2009. By 2018, an even greater 72% of samples contained over 150 milligrams of MDMA, with an average of 171 milligrams per pill — considerably higher than the average of 50-80 milligrams consistently seen in Europe across the 1990s and 2000s, and a steady rise from 2014. Recent years have also seen the rise of ‘superpills’- with a range of 270–340 milligrams — up to four times a normal adult dose. Rival producers, flush with low-cost raw materials, are competing with each other to market the strongest pills (even if, beyond a certain point, it is unclear whether this is something consumers actually want). The widening potential range of MDMA content in pills, combined with the emergence of super-high strength pills has been identified as a key driver in the rapid rise in MDMA-related medical emergencies and deaths since 2013. MDMA has also developed a substantial niche in online darknet markets accessed via dedicated TOR browsers and paid for using cryptocurrencies like Bitcoin. Estimates from darknet market studies in 2015 suggested that MDMA was the third most popular drug (after cannabis and pharmaceuticals) purchased on the darknet, accounting for 25% of drug sales. Of those who reported obtaining MDMA in the 2019 Global Drug Survey, 67% reported having obtained it through the darknet — higher than for any other drug. This is up from 48.7% in 2015, when the percentage was also higher than for any other drug. The EMCDDA also reported in 2019 that ‘transactions involving quantities of MDMA tablets indicative of the middle level of the market account for more than double the revenue of sales of retail-level quantities’. This is in stark contrast to other drugs sold on the darknet, like cannabis and cocaine, for which comparative sales are ‘overwhelmingly at the retail level’. User reports propose that MDMA purchased on the darknet is perceived to be better quality than supply from more conventional face-to-face dealer markets — perhaps in part because of the eBay-style user ratings system for products and vendors acting as an informal system of quality control and increased accountability of sellers. While concerns exist about the ease with which younger potential users might be able to access MDMA (and other drugs) via the darknet (the technical barriers to the market are relatively easily navigated by tech-savvy individuals), there may also be potential for reduced harm through informal quality controls and, for people without access to more established trusted sellers, reduced interaction with unknown dealers. As for MDMA legal status in Europe, depending on the country, there are differences in charges and legality of the substance. So, in the UK MDMA is classified as class A, charges for possession include the maximum of 7 years of imprisonment and/or indefinite term, lifetime for production and sale; Germany: illegal; France: illegal; Netherlands: illegal; Spain: illegal; Czech Republic: possession of 5 tablets and less is not considered a serious criminal offense. Portugal: the amount less than 1g is decriminalized. Other European countries: illegal. The USA: illegal, Schedule I class D 1995; Canada: Schedule III; Mexico: illegal; Australia: illegal; New Zealand: illegal; Singapore: illegal; Hong-Kong: illegal; Israel: illegal.
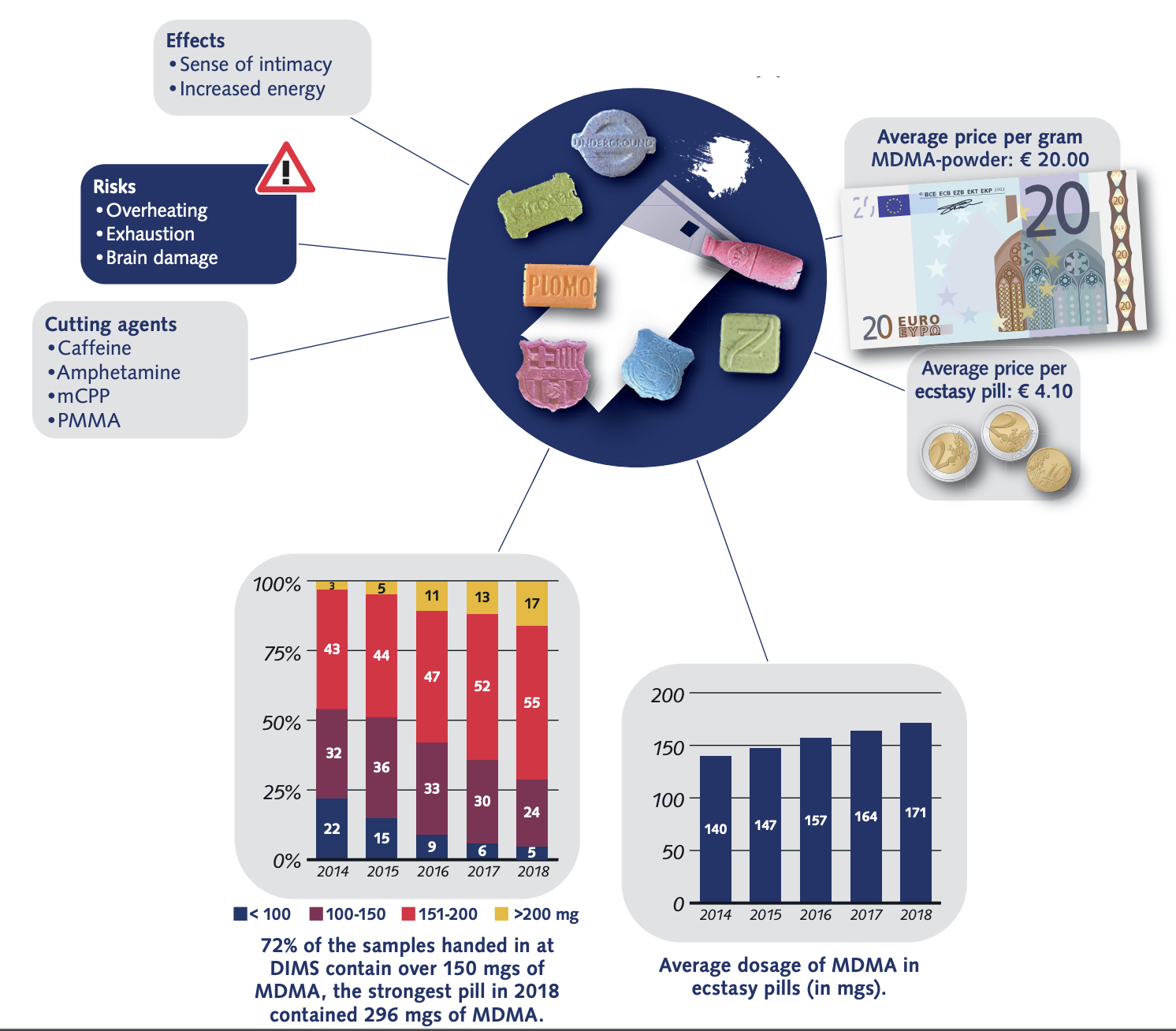
According to US Government DEA reports: in 2015, many drugs that were sold as MDMA/molly turned out to be synthetic cathinones, such as methylone or ethylone, as a replacement for the advertised drug. New Jersey: In 2014, reporting indicated that much of the MDMA being trafficked in New Jersey was actually methylone. True MDMA was too expensive to make a profit, so methylone was substituted. New York: In 2014, laboratory analyzis showed most of the purported pure MDMA/“molly” contained cathinones such as methylone. 87% of “Molly” analyzed by the DEA between 2009 and 2013 contained 0% MDMA, instead mostly containing “bath salts” like methylone. In West Florida, 0% of analyzed “Molly” contained any MDMA, also instead mostly containing “bath salts.” “Laboratory analyses on drug seizures by DEA in New York and submitted as Molly between 2011 and 2012 revealed the exhibits were actually a variety of controlled and noncontrolled substances, such as 3,4-methylenedioxymethcathinone (methylone), 4-methyl-n-ethylcathinone (4-MEC), 3,4-methylene-dioxymethamphetamine (MDA), and 3,4-methylene-dioxyprovalerone (MDPV) but not MDMA”. As of 2017, Ecstasy pills in the US have been relatively pure, with most Ecstasy pills sold in the US now containing primarily MDMA. Some pills, particularly in Europe are dangerous as they contain real MDMA, but at too high/unsafe dosages.
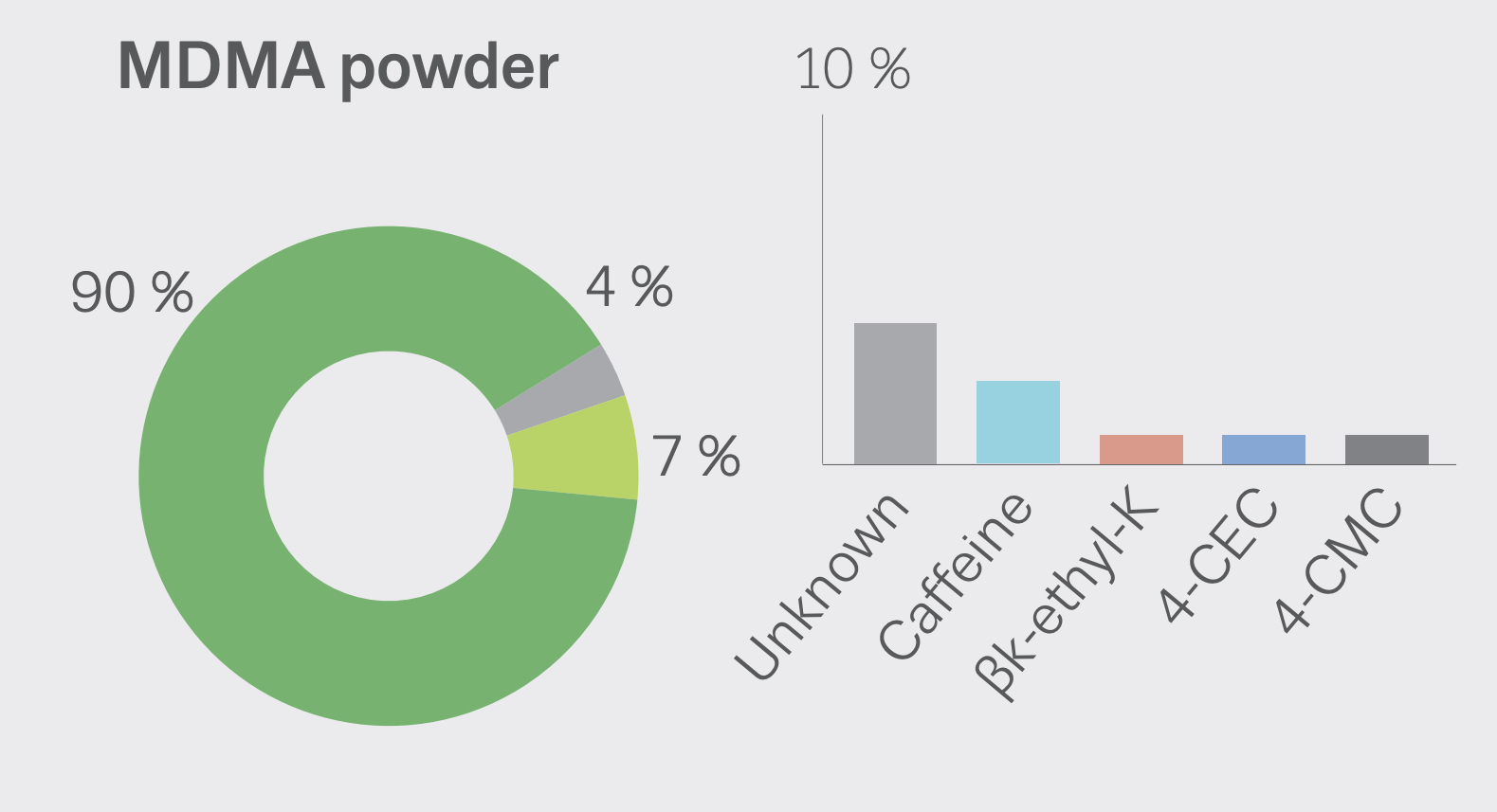
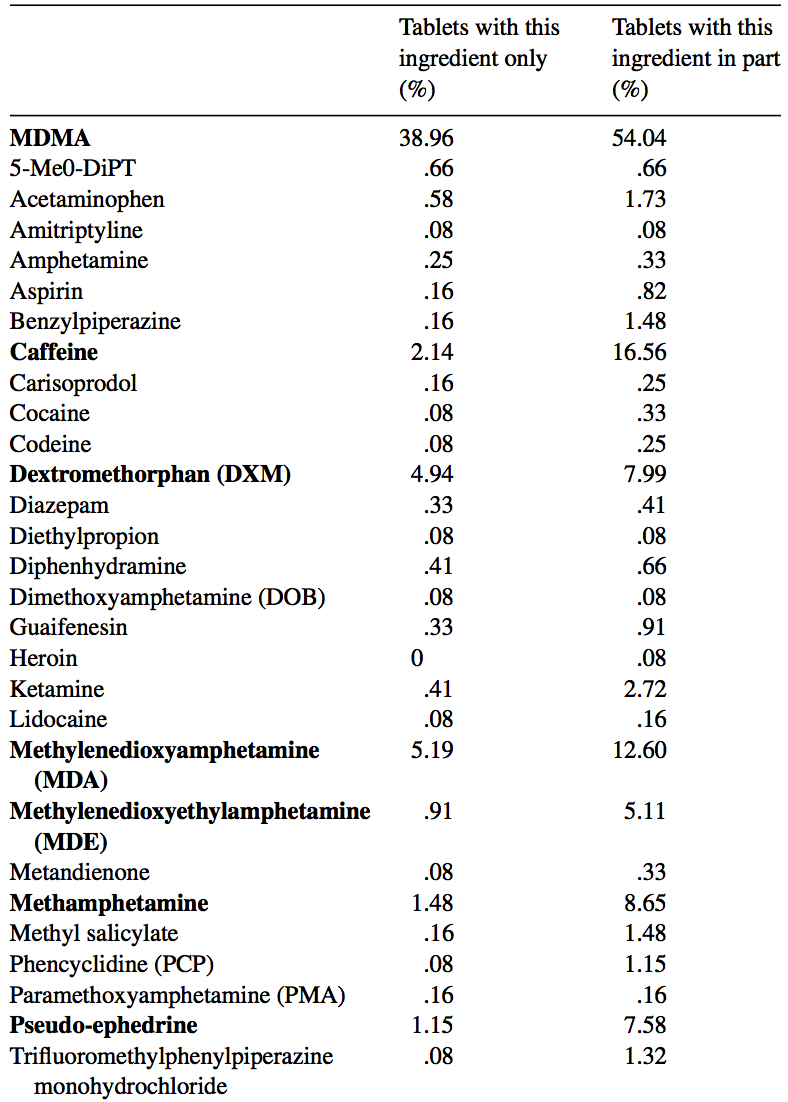
MDMA is structurally similar to amphetamines and mescaline. However, it is functionally different. This substance has a molecular formula C11H15NO2 named (RS)-1-(Benzo[d][1,3]dioxol-5-yl)-N-methylpropan-2-amine. MDMA is a chiral molecule, which has two enantiomers S and R. S-MDMA has more powerful effects than R-MDMA. According to research data, S-form is associated with a release of large amounts of dopamine, while R-form increases the level of serotonin. The substance is most often found in the form of hydrochloride salt, which is easily soluble in water at pKA of 9.9. This fact underlies its ionization in plasma. The substance is lipophilic and passes through the blood-brain barrier. As a result of the research on MDMA, it was revealed that it is a rather stable compound, which could remain clinically active even after 21 years of storage. Considering the fact, there aren't any special conditions for storage, it is recommended. However, to store in closed, airtight containers, without ultraviolet light exposure at ambient temperature. Boiling point is at 100-110 °C at 0.4 mmHg. Water-solubility is 7034 mg/L at 25 °C (est), vapor pressure 2.27X10-3 mm Hg at 25 °C (est). As a rule, ecstasy can be found in form of hydrochloride, which has an appearance of powder from white to brown colour, soluble in water (it is not recommended using the precipitate which forms most of the time), and can be put in gelatin capsules for oral administration. The most common form is pressed tablets of different shapes and colours. Frequently, tablets contain other substances and impurity, which can be either other psychoactive substances or adulterants without any psychoactive action. The variety of adulterants in tablets is huge and can range from caffeine to 2C-В, affecting qualitative and quantitative characteristics. As a rule, average concentration of MDMA in tablets ranges from 75% to 95%. Sadly, a 2005 paper found that 61% of tested ecstasy tablets were adulterated with other drugs, in part due to the lack of regulation of the illegal market. And a massive 46% of Ecstasy pills contained 0% MDMA. 39% of Ecstasy pills contained only MDMA, 5% of Ecstasy pills contained only MDA (similar substance to MDMA, though far less studied), 5% of Ecstasy pills contained only DXM (typically found in Robitussin, the cough medicine), 2% contained only caffeine, 1% contained only methamphetamine, 1% contained only psuedo-ephedrine (a stimulant found in cold and flu medicine), and the rest were unknown or mixed.
Pharmacokinetics and pharmacodynamics.
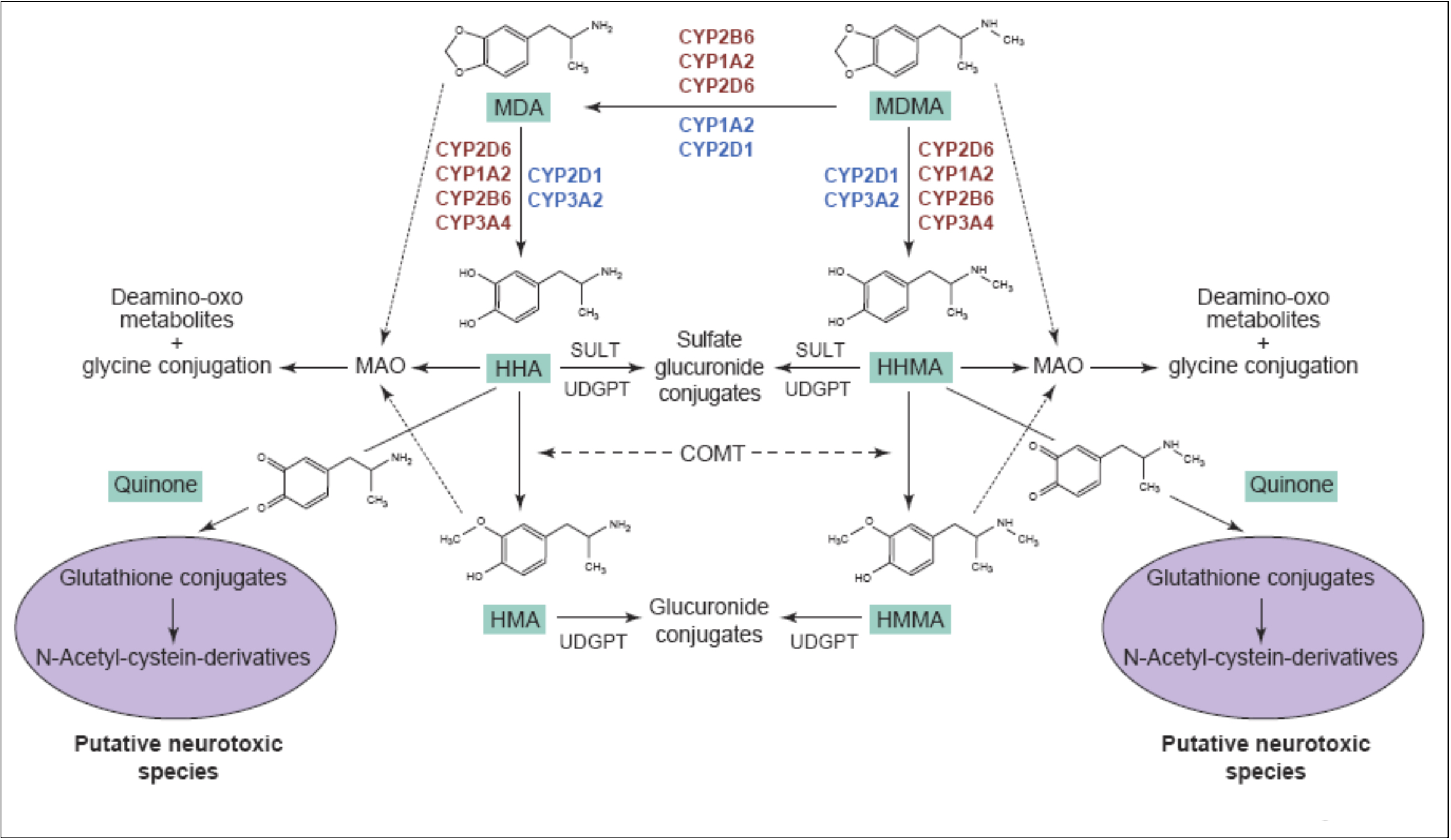
The primary routes for metabolism of MDMA are N-demethylation and loss of the methylene bridge connecting the catechol, both of which are mediated by various cytochrome P450s. The common metabolites of MDMA include MDA, 3,4-dihydroxymethamphetamine, 3,4-dihydroxyamphetamine, 4-hydroxy-3-methoxy-methamphetamine, and 4-hydroxy-3-methoxy-amphetamine. The
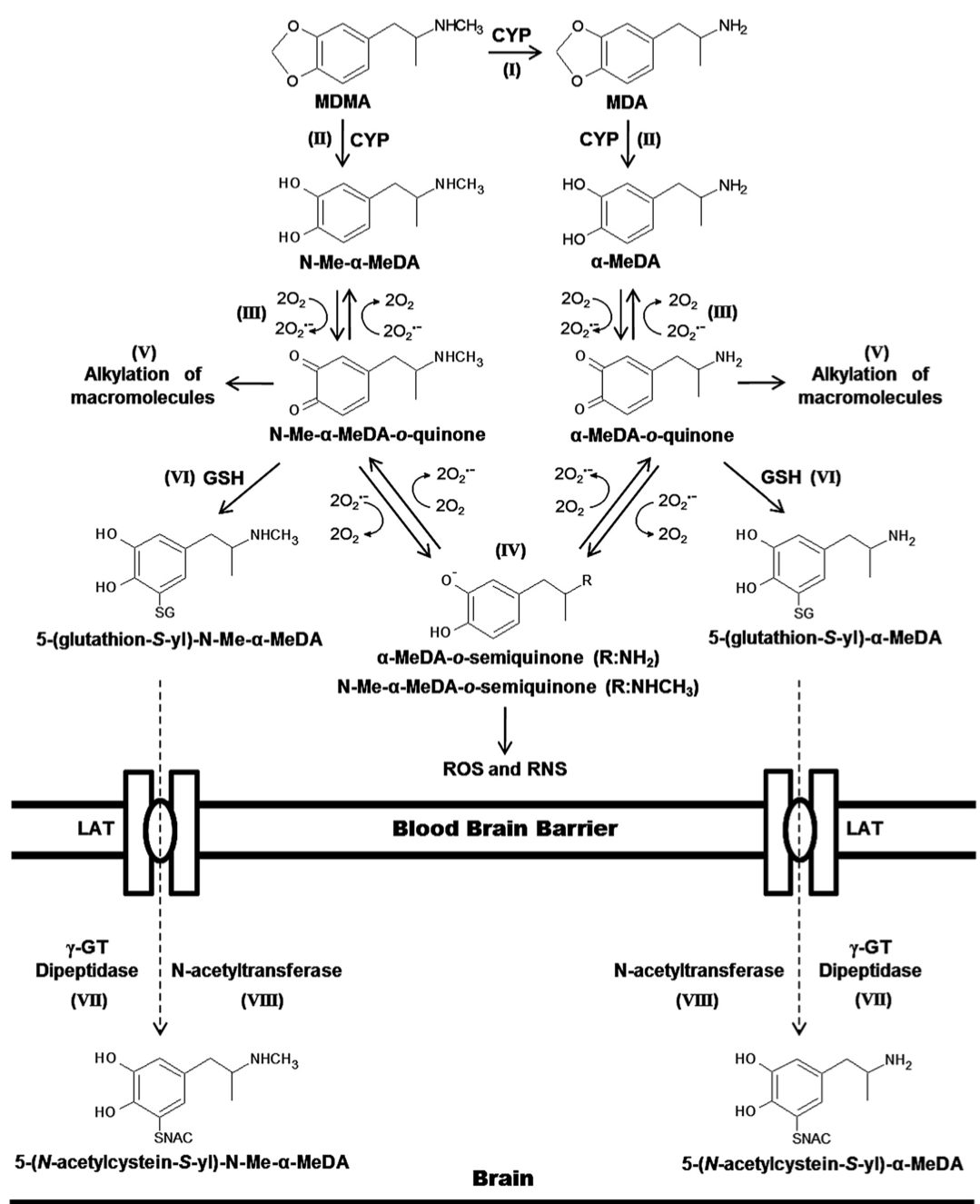
major metabolite of MDMA in humans is HMMA, which is mainly excreted as the glucuronic acid conjugate. Recent genetic findings suggests that a variety of cytochrome P450s, including CYP2C19, CYP2B6, and CYP1A2, play a role in the demethylation of MDMA. Mutations in the CYP2C19 or CYP2B6 genes that reduce enzyme function have been shown to increase the ratio of MDMA/MDA but do not alter HMMA concentrations. Subjects with decreased CYP2C19 function also showed greater cardiovascular responses with faster onset times. Mutations in the CYP2B6 gene resulting in decreased enzyme function only influenced metabolism at later time points (i.e., 3−4 h) suggesting that it is a secondary metabolizer of MDMA. When MDMA is administered to humans at a dose of 100 mg, it has a half-life of approximately 8−9 h and yields plasma Cmax and tmax values of 222.5 ng/mL and 2.3 h, respectively. Median lethal dose for human is about 10-20 mg/kg. As a rule, the onset of MDMA effects starts after 20-30 minutes and the effects last for a couple of hours, while the peak of action is at 70-120 minutes. It is important to note, that the intake of a second dose (which exceeds the initial by two times) doesn’t lead to any significant increase in duration and intensity of effects. Half-life period of MDMA after oral administration is 7-8 hours, it increases with repeated administration. Metabolites, which are presented in the table, are excreted mainly in the forms of glucoronide and sulfate conjugates, stereoselective metabolism has also been proven. MDMA and its only active metabolite MDA are present in saliva in higher concentrations, than in plasma, at concentration value of 1-1.6 mg/kg. MDMA is metabolized in liver by a number of p450 cytochrome enzymes, including CYP1A2, CYP3A4, and CYP2D6. It has been proved, that MDMA inhibits 2D6 function in high doses. Its activity is usually restored within ten days. Different genotypes of CYP2D6 don't have any clinical significance. MDMA causes an increase in CYP1A2 activity, as evidenced by the comparison of caffeine metabolism before and after intake of MDMA; according to the research, variants with less functional versions of genotypes CYP2C19 and CYP2B6 demonstrate higher maximum concentration of MDMA in plasma, which induces more pronounced cardio-vascular reaction to the substance. COMT and monoamine oxidase are the enzymes which may be involved in the metabolism of the substance. At least one of the variations of COMT affects both elimination rate of MDMA and systolic blood pressure after use of the substance. Combination of MDMA with monoamine oxidase inhibitor (MAOI) highly potentiates the risk of serotonin syndrome development and of increase in sympathetic activity. A retrospective analysis revealed a big number of lethal cases due to this fact, as well as non-lethal cases of serotonin syndrome. As a result of the studies on MDMA effect on serotonergic system, an increase in cumulative level of MDMA in 5-HT1A variant of the receptor and a mild decrease in maximum concentration in one of the 5-HT1B variants have been found, however, these changes are clinically insignificant.

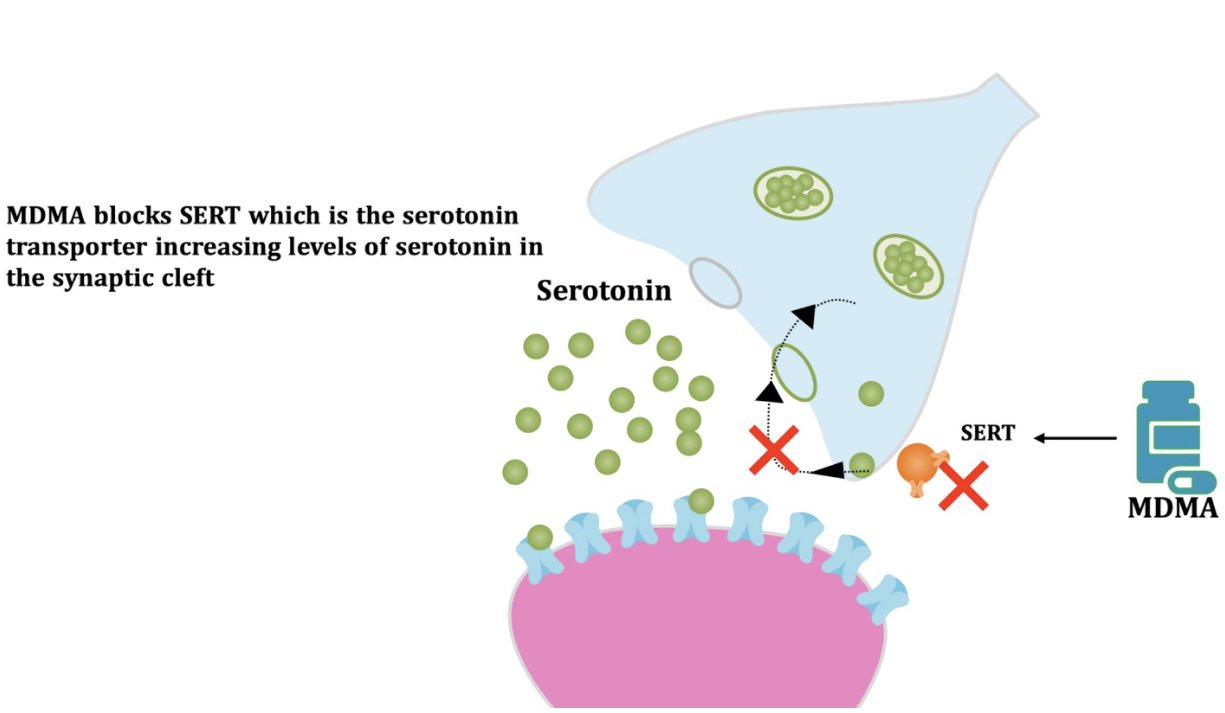
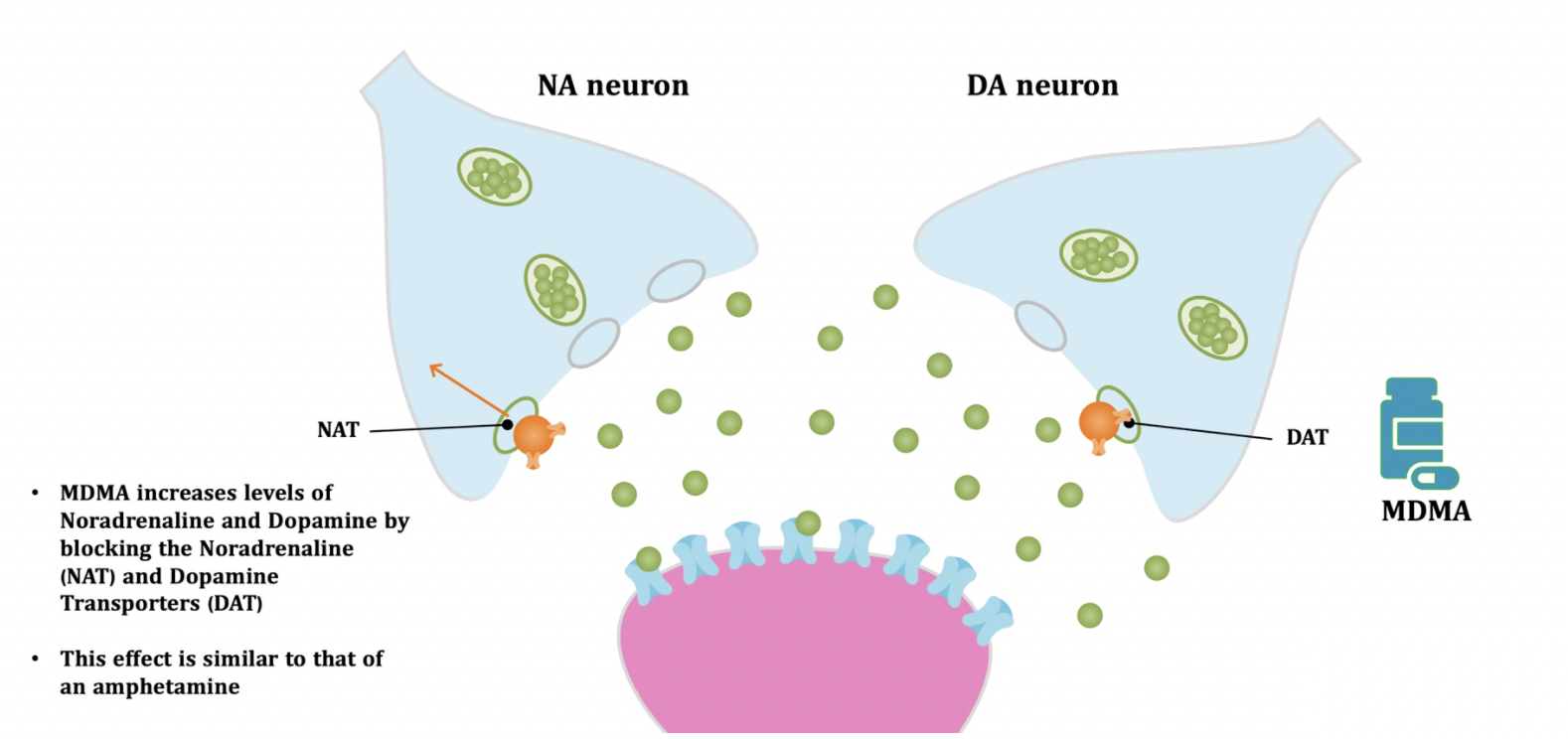
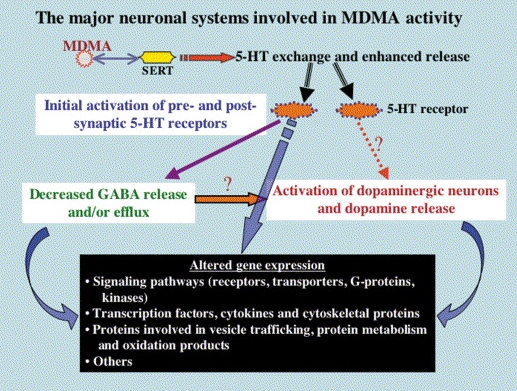
Pharmacodynamic characteristics of MDMA involve release, inhibition of reuptake of serotonin, norepinephrine and dopamine in the synaptic cleft. Generally, MDMA belongs to a unique class of psychoactive substances named entactogens, which are considered to cause changes in mood and social interactions with a sense of intimacy. Firstly, the substance binds to and inhibits SERT,
DAT and NET transporters, decreasing monoamine reuptake, which leads to an increase in the extracellular level of these amines. Inhibition occurs due to the fact that MDMA acts as a substrate, not blocker of these transporters, unlike, for example, amphetamine. Also, MDMA prevents transport of monoamines into vesicles, but it doesn't affect cellular uptake or "vesiculation“ of GABA or glutamate. MDMA binds to a number of neuroreceptors, including adrenergic, serotonergic, histamine and muscarine. That is why the idea of MDMA causing most of these effects “indirectly” and modulating monoamine levels is associated with micromolar affinity for these various receptors. Micromolar binding affinity of MDMA to 5-HT2A-receptors is associated with certain psychodelic effects, but theoretically not in all people. TAAR1 has been identified as a key target of MDMA agonistic activity, increasing cAMP level. It is interesting to note that 4-hydroxy-substituted is a powerful TAAR1 agonist. Radioligand binding studies have shown that MDMA binds to both sigma-1 and sigma-2 receptors with Ki values in the low micromolar range, which are comparable to the affinities of MDMA for monoamine transporters. Moreover, treatment with BD1063, a selective sigma-1 antagonist, blocked the effects of MDMA on rodent locomotion. The sigma-1 receptor has been proposed to be a novel target for the treatment of depression and anxiety, and it is reasonable to hypothesize that this receptor plays some role in the behavioral and clinical effects of MDMA. Binding affinity of MDMA for adrenergic receptors is low, but since MDMA increases NE levels via transporter-mediated NE release and NET uptake inhibition, indirectly NE-mediated effects at adrenergic receptors clearly contribute to MDMAaction. β-Adrenoceptors are involved in MDMA-induced increase in heart rate. The α1- and β-adrenoceptors have been implicated in hyperthermia and drug-induced vasoconstriction. α2-Adrenocepors are associated with sympathomimetic toxicity and augmented NE release. Potent transporter-mediated NE release or even NET inhibition seems sufficient to induce cardiostimulant effects mediated through the different adrenergic receptors. NPS with potent effects at NET thus likely induce psychostimulation and sympathomimetic toxicity. Administration of MDMA to humans leads to robust increases in plasma levels of cortisol, prolactin, dehydroepiandrosterone (DHEA), vasopressin, and oxytocin. It is possible that some of these hormonal changes are the result of serotonergic activity, and it is likely that they modulate some of the effects of MDMA. For example, the rise in plasma DHEA levels was significantly correlated with feelings of euphoria. Furthermore, the effects of MDMA on oxytocin levels are often invoked to explain the drug’s prosocial effects.
Dumont and co-workers were the first to demonstrate in a controlled laboratory setting that MDMA increases oxytocin levels. They also found that increases in blood oxytocin levels were correlated with the subjective prosocial feelings induced by MDMA more so than blood levels of the drug itself. While numerous other studies have replicated the finding that MDMA elevates oxytocin levels,
they have all failed to reproduce a correlation between oxytocin levels and prosocial feelings, calling into question the relevance of this hormone for the prosocial effects of MDMA. As such, the role of oxytocin in the effects of MDMA is currently controversial. Like other serotonergic psychedelics, MDMA produces behavioral effects consistent with serotonin syndrome such as flat body posture, hind limb abduction, and forepaw treading. At lower doses, MDMA produces “amphetamine-like” hyperactivity in the open field. Both of these effects are enhanced following repeated administration of MDMA, demonstrating that MDMA is capable of producing behavioral sensitization. Behavioral sensitization is correlated with the enhanced ability of MDMA to increase monoamine levels (measured via microdialysis) following repeated dosing. The loco motor effects of MDMA are perhaps the best-studied behavioral responses in rodents, and they are modulated by a variety of neuroreceptors including 5-HT1B, 5-HT2A, D1, and D2 receptors. Unlike amphetamine, selective serotonin reuptake inhibitors block MDMA-induced increases in locomotion. Furthermore, MDMA does not produce this behavioral effect in mice genetically lacking SERT, further implicating this monoamine transporter in the hyperlocomotive effects of MDMA. In rodent models of anxiety, MDMA produces complex effects. At low acute and subchronic doses, MDMA tends to be anxiogenic in the elevated plus maze (EPM). However, at higher acute and subchronic doses, MDMA produces anxiolytic effects in the EPM. When tested in the light-dark box paradigm, MDMA does not alter preferences of mice for the two compartments. Though racemate MDMA is the most common form, which is used both recreationally and in various preclinical and clinical trials, there is a significant difference between the two enantiomers. S-enantiomer of MDMA is a more powerful compound; however, R-enantiomer has higher affinity to 5-HT2A receptor, which presumably explains its tendency to cause perception impairment. Neither enantiomer is particularly effective at stimulating phosphatidyl inositol turnover in either 5-HT2A or 5-HT2C expressing cells. When rats were trained to discriminate S-amphetamine, LSD, and saline from each other in a 3-lever drug discrimination paradigm, R-MDMA and S-MDMA produced more hallucinogen-like and amphetamine-like discriminative stimuli, respectively. Furthermore, experiments using mice trained to discriminate either S-MDMA or R-MDMA from vehicle demonstrated that the S-enantiomer produced more psychostimulant-like effects while the R-enantiomer was more hallucinogen-like. In terms of their influences on hormone levels, the enantiomers of MDMA also have differential effects. Ex vivo studies utilizing rat hypothalamus tissue demonstrated that S-MDMA is a more potent inducer of oxytocin release than the racemate, while R-MDMA has no effect. However, R-MDMA was more effective at increasing the activation of hypothalamic oxytocinergic neurons, as measured by the number of c-fos positive neurons. Both enantiomers appear to increase vasopressin secretion comparably from the hypothalamus ex vivo. R-MDMA more potently increased plasma prolactin levels in rhesus macaques. Pretreatment with fluoxetine attenuated this effect, but did not block it completely. The selective 5-HT2A antagonist M100907 was required to completely inhibit R-MDMA- induced increases in prolactin, suggesting that indirect effects on 5-HT levels, as well as direct binding to 5-HT2A receptors contribute to the ability of R-MDMA to increase prolactin levels.
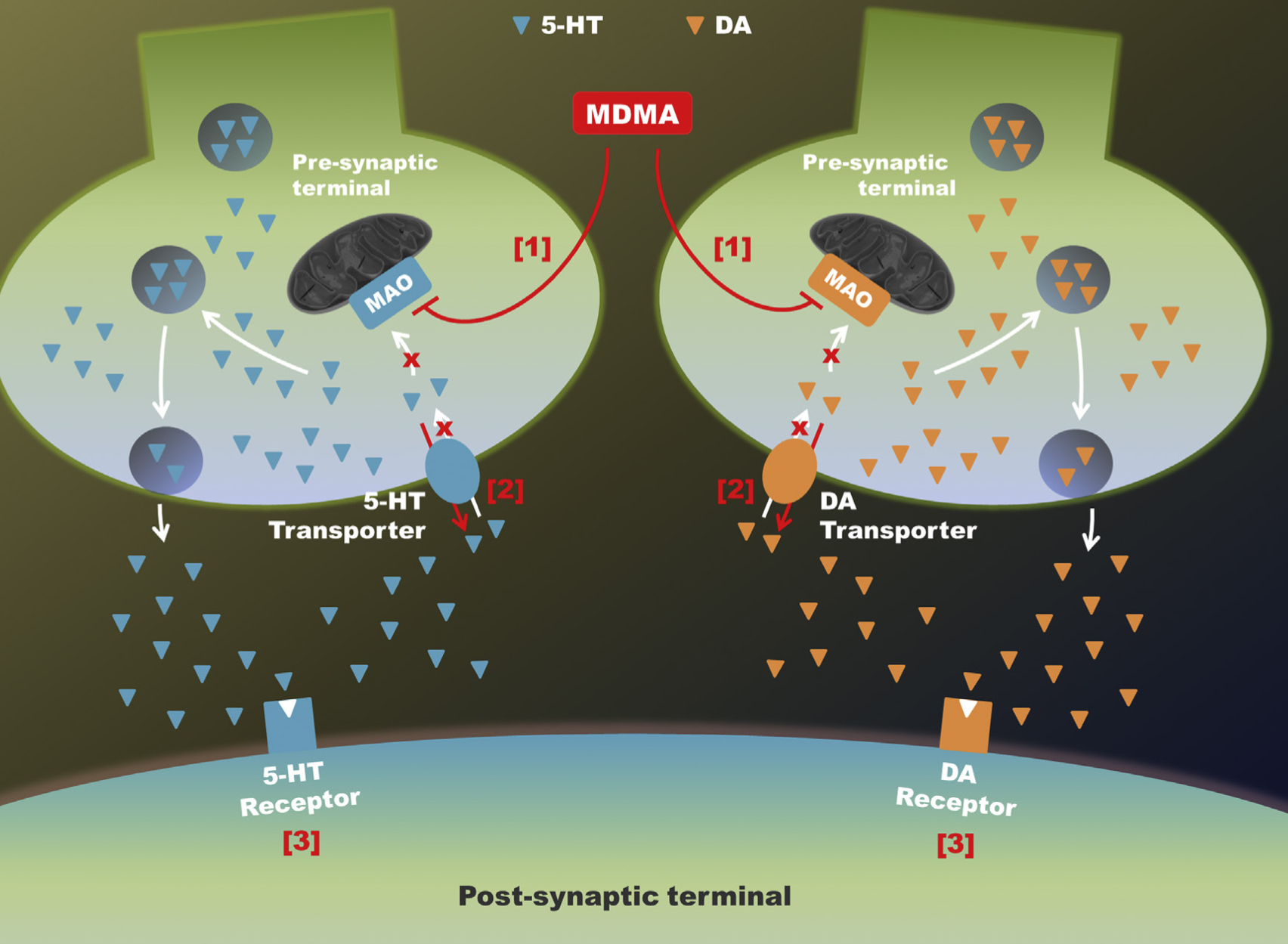
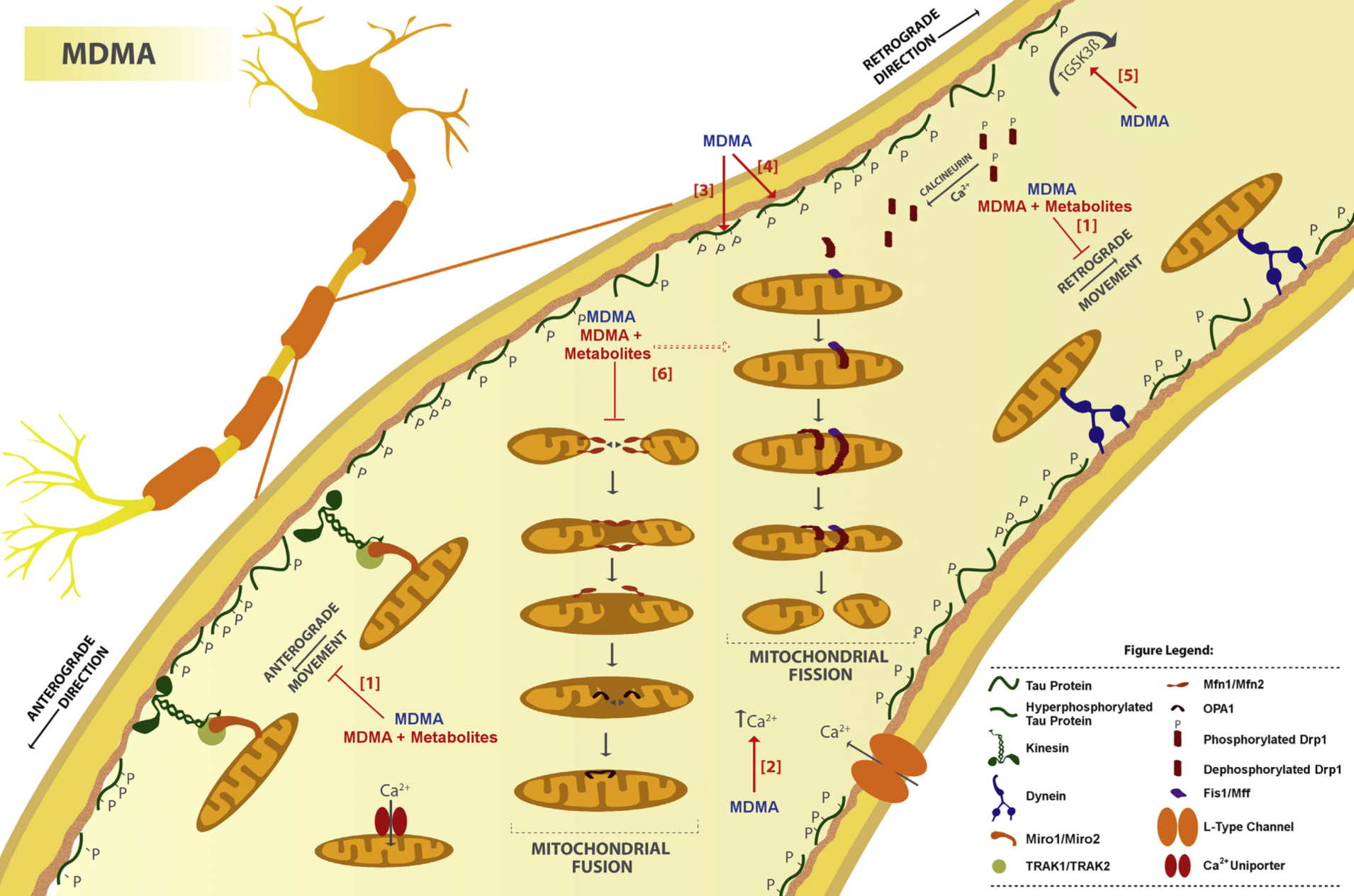
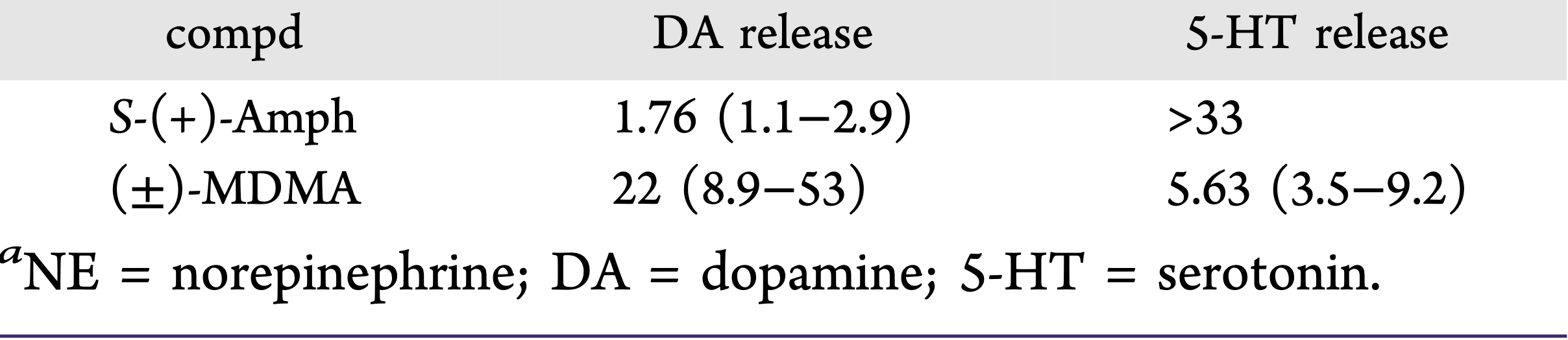
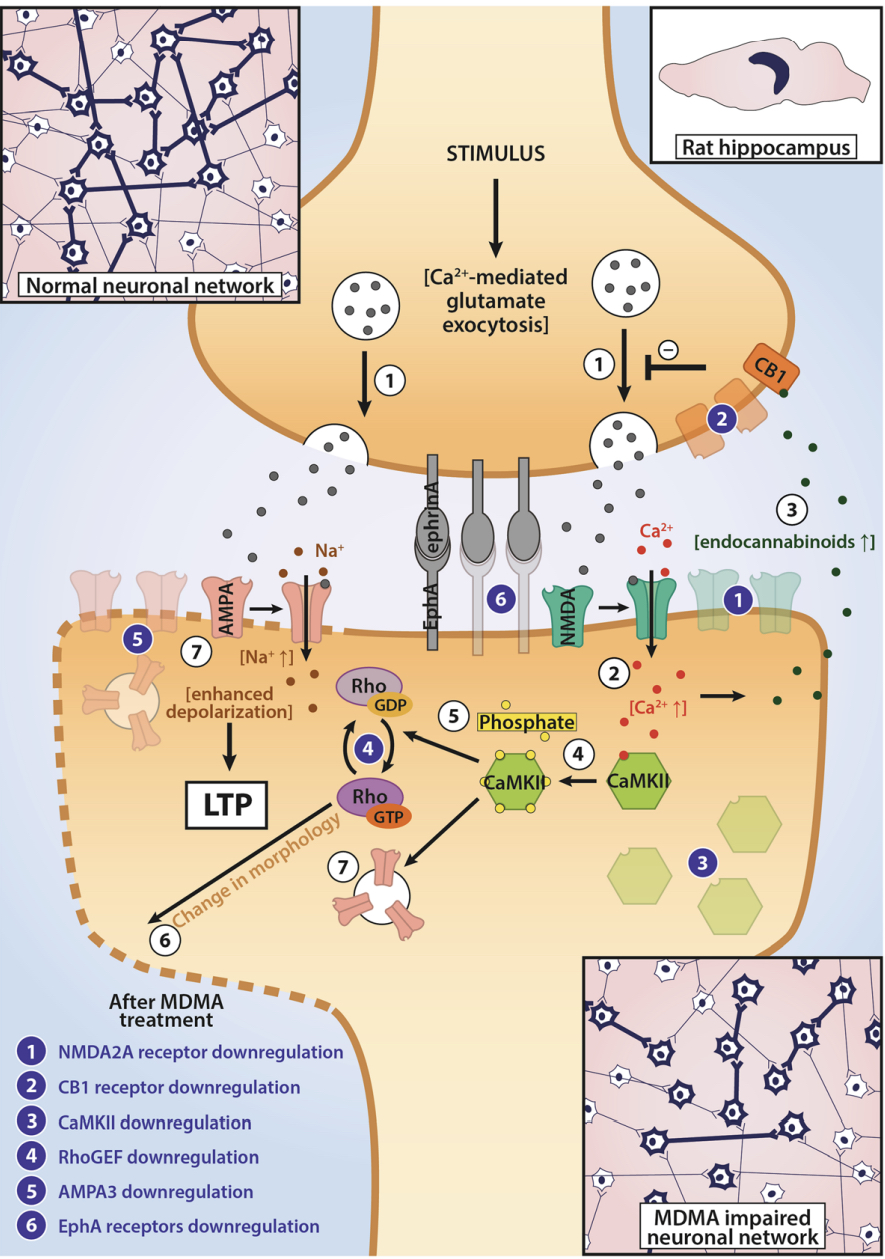
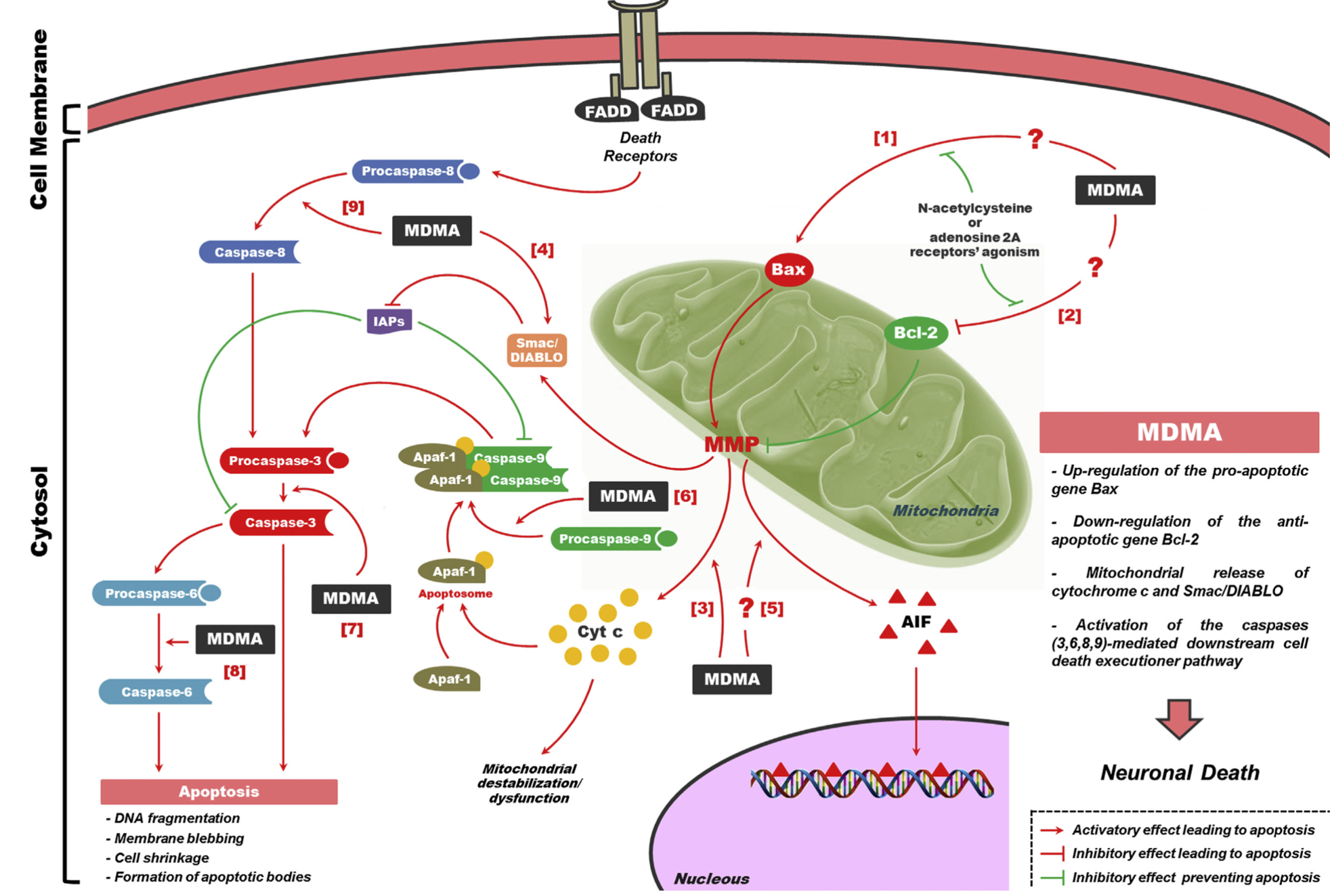
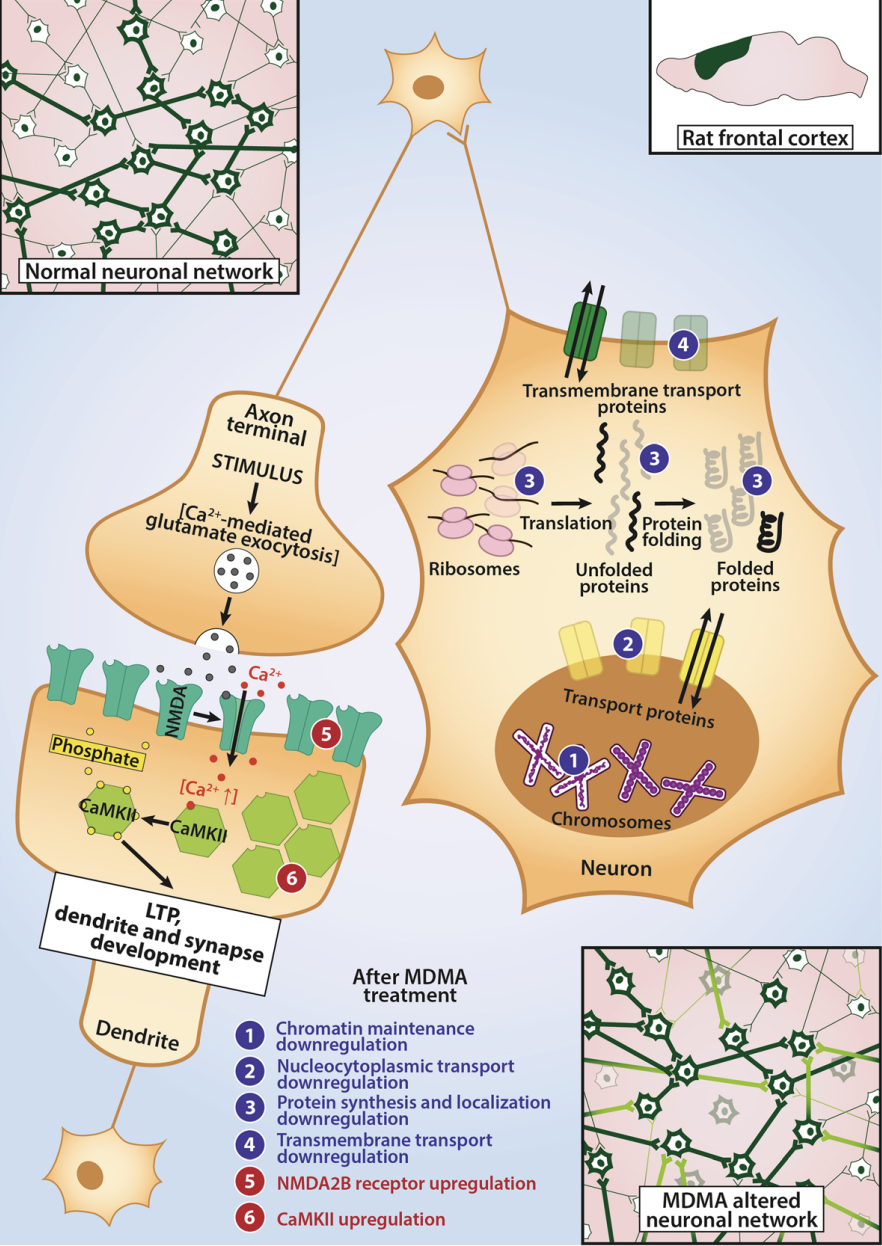
A lot of data obtained as a result of using human monoamine transporters expressed in cells, shows higher affinity of MDMA to NET, than to serotonin or dopamine transporter. MDMA induces more detectable release of serotonin comparing to, for instance, norepinephrine. This fact indicates the importance of both systems, regardless of the degree of affinity for certain receptors. Since NET has higher affinity to dopamine than DAT, it is predominantly expressed in the brain areas, where NET concentration is higher, for example, in frontal cortex. The relative affinities of MDMA for various monoamine reuptake transporters, and the affinity of the respective transporters for each neurotransmitter, can thus influence the selectivity of signaling pathways MDMA activates in a region-specific manner depending on transporter density and availability. Some of MDMA effects (e.g. the level of anxiety or mood) correlate to dopamine release, since there is evidence consisting of studies, which involved pretreatment with dopamine receptors antagonist. Surprisingly, methylphenidate doesn't enhance or reduce MDMA effects, when used together with the latter. Disrupted calcium homeostasis and depletion of cAMP in neurons occurring after use of MDMA, allows assuming that its metabolites affect mitochondrial dynamics. So, impaired mitochondrial events regulation in neurons of hippocampus (which express Mfn2, Mfn2 R94Q) indicates an impairment in their "transfer" and an increase in fragmentation. Thus, this information gives an idea about the main aspects of negative neurotoxic effect of this substance. When performing a PET against the background of MDMA use, there is a decrease in the activity of the left amygdala and an increase in the activity of the frontal part; regional cerebral blood flow (rCBF) increase in the ventromedial prefrontal and cerebellar regions, and a decrease in this indicator in the left amygdala. An activity decrease in the amygdala may indicate a decrease in responding potential threats. Also, during functional MRI, a weakening of activity in the left anterior temporal region is detected, which may increase the probability of “negative” or “undesirable” memories during ecstasy use. According to the studies on MDMA effects on immune system, there is a decrease in CD4 cells, a decrease in the CD4/CD8 ratio, inhibition of lymphocyte proliferation in response to mitogen and an increase in the number of NK cells. The effects level over time, but within 24 hours it remains. Also, ecstasy reduces the production of pro-inflammatory cytokines, including IL-6, IL-1, TNF и INF, and increases the production of anti-inflammatory cytokines, including IL-10 and TGF-ß. Generally, MDMA reduces the concentration of Th1 cytokines and increases the concentration of Th-2 cytokines. Based on the study findings, MDMA causes an obvious increase in body temperature with a certain influence of ambient temperature.