G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,704
- Solutions
- 3
- Reaction score
- 2,845
- Points
- 113
- Deals
- 1
Introduction
In this thread, I want to represent two ways of acetic anhydride. Moreover, there is acetyl chloride synthesis, which used as precursor in one of mentioned above synthesis. The first way of acetic anhydride synthesis carry out from sodium acetate and acetyl chloride. This method has some disadvantages: you have to produce acetyl chloride (if you can't buy it); acetyl chloride quite unpleasant substance with strong odor and has toxic effects. In the other hand, this way easy to implement in case if you have opportunity to buy acetyl chloride. The second way has a great yield, but you have to use disulfur dichloride, which is quite exotic reagent. But in this topic you may find special manual to produce sulfur chloride in situ, which requires an apparatus for the production of chlorine gas. Also, you can find video manual of sodium acetate synthesis (easy to obtain from acetic acid and baker soda) at the end of the topic.
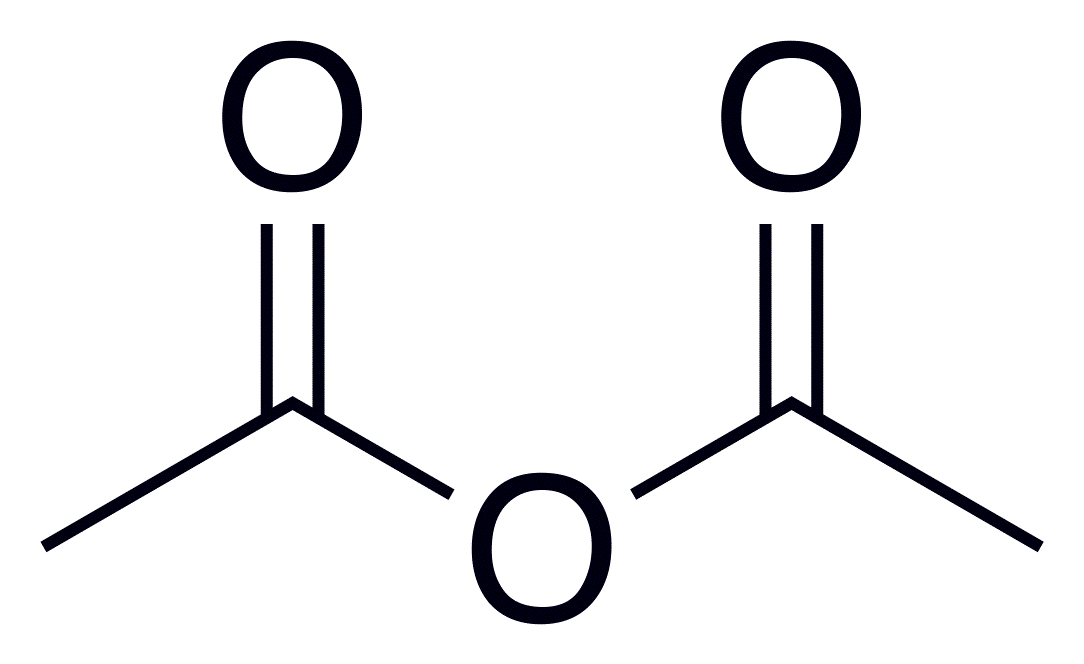
Appearance: colorless liquid with strong odor;
Boiling Point: 139.8 °C/760 mm Hg;Appearance: colorless liquid with strong odor;
Melting Point: -73.1 °C;
Molecular Weight: 102.089 g/mol;
Density: 1.082 g/ml (20 °C);
Refractive Index: 1.3901 at 20 °C/D.
Acetyl chloride synthesis
Equipment and glassware:
- Three-necked round bottom flask, 500 ml;
- Round bottom flask, 500 ml;
- CaCl2 Tube;
- Drip funnel, 50-100 ml;
- Water bath;
- Erlenmeyer flask, 100 ml x2 (with cap);
- Distillation apparatus;
- Retort stand and clamp for securing apparatus;
- Laboratory grade thermometer (0 °C to 100 °C) with flask adapter;
- Laboratory scale (0.1 - 200 g is suitable);
- Measuring cylinder, 50-100 ml.
Reagents:
- Phosphorus chloride 5 (PCl5) 57 g;
- Glacial acetic acid 80 g;
- Dimethylformamide 7 ml (dried over MgSO4).
In a round-bottom three-necked flask 500 ml, placed in a water bath with cold water, we place all the phosphorus chloride (PCl5), then we install a reflux condenser with a CaCl2 tube, a thermometer and a drip funnel. Pour glacial acetic acid 40 g into the drip funnel. We begin to slowly add the acid. The reaction proceeds violently with the release of hydrogen chloride (HCl). Mix the second portion of acetic acid (40 g) with dimethylformamide (DMF) in a 100 ml Erlenmeyer flask. The solution of DMF in acid was added slowly to the reaction mixture through dropping through a dropping funnel. After adding all acetic acid (the first and second parts), the reaction mass is boiled for 20 minutes at 80 °C. Next, cool the reaction vessel to room temperature.
The reaction mass is distilled in a fractional distillation apparatus in a water bath. We collect the fraction boiling at 50-55 °C. When the boiling point rises above 55 °C, the distillation should be finished. Acetyl chloride attacks corks and rubber stoppers. The yield is 20 %.
Acetic anhydride from sodium acetate and acetyl chloride
Equipment and glassware:
- Three-necked round bottom flask 250 ml;
- Mantle or oil bath;
- CaCl2 Tube;
- Drip funnel, 50-100 ml;
- Erlenmeyer flask, 100 ml x2 (with cap);
- Distillation apparatus;
- Retort stand and clamp for securing apparatus;
- Laboratory grade thermometer (0 °C to 100 °C) with flask adapter;
- Laboratory scale (0.1 - 200 g is suitable);
- Measuring cylinder, 50-100 ml;
- Boiling stones.
Reagents:
- Sodium acetate (NaOAc) 60 g;
- Acetyl chloride 40 g (36 ml).
The precautions for exclusion of moisture must be observed. In a 250 ml round bottom flask, place 60 g of finely pulverized anhydrous sodium acetate (*). Arrange in place the condenser, dry receiving flask and drying tube; the receiver need not be cooled. Check to ensure that all connections are tight. Cool the reaction flask in a bath with cold water and add dropwise, through the drip funnel, 40 g (36 ml) of acetyl chloride.
After the addition has been completed, remove the water bath and shake the flask to obtain good mixing of the reactants. Recheck the connections for tightness. Dry the outside of the flask with a towel and heat it with a mantle or oil bath. Continue the heating until no more distillate comes over but do not overheat the solid residue.
To the distillate add 4-6 g of finely powdered anhydrous sodium acetate, to react with a small amount of acetyl chloride that may be present. Add a boiling chip and redistill the crude acetic anhydride. Collect the product distilling at 138-141 °C in a dry flask. The yield is 30-40 g (57-77%).
(*) Note: Commercial anhydrous sodium acetate usually contains some moisture. To remove moisture, fuse 70-80 g in a casserole (either in an ordinary oven, or a microwave) and stir until no more water is evolved. Do not overheat! Cool and quickly pulverize. Store in a tightly sealed container before use.
To the distillate add 4-6 g of finely powdered anhydrous sodium acetate, to react with a small amount of acetyl chloride that may be present. Add a boiling chip and redistill the crude acetic anhydride. Collect the product distilling at 138-141 °C in a dry flask. The yield is 30-40 g (57-77%).
(*) Note: Commercial anhydrous sodium acetate usually contains some moisture. To remove moisture, fuse 70-80 g in a casserole (either in an ordinary oven, or a microwave) and stir until no more water is evolved. Do not overheat! Cool and quickly pulverize. Store in a tightly sealed container before use.
Acetic anhydride from sodium acetate and disulfur dichloride (S2Cl2)
Equipment and glassware:
- Three-necked round bottom flask 1000 ml;
- Beakers 100 ml x2, 1 l x2;
- Glass rod;
- Water bath;
- Heating plate;
- Distillation apparatus;
- Retort stand and clamp for securing apparatus;
- Laboratory grade thermometer (0 °C to 200 °C) with flask adapter;
- Laboratory scale (0.1 - 500 g is suitable).
Reagents:
- Sodium acetate (NaOAc) 400 g;
- Disulfur dichloride (S2Cl2) 260 g (or S2 and Cl2 gas);
- KMnO4 or K2Cr2O7 (few grams).
Prepare 100 g freshly fused NaOAc and 65 g disulfur dichloride (S2Cl2). A small quantity of sodium acetate (NaOAc) is placed in a baker, cooled in an ice bath. To this is added some sulfur dichloride, the mixture is vigorously stirred with a glass rod, not allowing the temp to rise. Then some NaOAc is added again, and the process is repeated several times until all is mixed in. The semi-liquid mass is transferred into a 1 liter three necked round bottom flasks. The previous operation is repeated 4 times, so that 400 g NaOAc and 260 g S2Cl2 total are taken into work.
The round bottom flask is then equipped with a reflux condenser and gently heated on a water bath to ~80-85 °C. As soon as the reaction starts, the heating is removed, and in case the reaction gets too vigorous it's cooled with cold water.
After 20-30 min, SO2 evolution ceases and the mixture is heated for 10 more minutes on a boiling water bath. The reaction product is then distilled off under vacuum, then fractionally re-distilled at atmosphere pressure, collecting the fraction boiling between 138-141 °C.
For further purification it's distilled with 2-3 % KMnO4 or K2Cr2O7 for breakage of sulfurous contaminants (test for their presence: 1 ml of the distillate upon neutralization with pure NH3 mustn't give a dark precipitate on treatment with Pb(AcO)2) The yield is ~90 % based on S2Cl2.
For further purification it's distilled with 2-3 % KMnO4 or K2Cr2O7 for breakage of sulfurous contaminants (test for their presence: 1 ml of the distillate upon neutralization with pure NH3 mustn't give a dark precipitate on treatment with Pb(AcO)2) The yield is ~90 % based on S2Cl2.
Another way with generating sulfur chloride in situ
Mix quickly and thoroughly 205-215 g of pulverized fine sodium acetate (NaOAc) and 10 g dry sulfur powder (S2), the mixture is quickly transferred to a wide-mouth 1 liter three necked round bottom flasks and wetted with 25 ml acetic acid. Into the flask through the rubber cork extend 1) a wide tube for chlorine in-flow 2) an overhead stirrer, which is sealed with a piece of rubber tubing greased with Vaseline and 3) an out-leading tube for chlorine (Cl2) excess release. The flask is immersed in an ice-bath. Chlorine is initially passed in cautiously, with frequent stirring or shaking, over the passage of time the reaction gets hotter and more and more liquid, so the stirrer may be after some time rotated with a motor. Chlorine stream should be regulated so that almost all of it is absorbed. When the reaction mixture stops heating and Cl2 is no longer taken up, the reaction contents are distilled in vacuo at oil bath temp ~150-180 °C, then re-distilled at ordinary pressure, collecting the fraction boiling between 138 and 141 °C. Yield ~90 %.
Sodium acetate (NaOAc) synthesis
Last edited:
