- Joined
- Jul 5, 2021
- Messages
- 3,323
- Solutions
- 3
- Reaction score
- 3,807
- Points
- 113
- Deals
- 1
Introduction
Suction filtration (vacuum filtration) is the standard technique used for separating a solid-liquid mixture when the goal is to retain the solid (for example, in crystallization). Similar to gravity filtration, a solid-liquid mixture is poured onto a filter paper, with the main difference being that the process is aided by suction beneath the funnel (Fig.1).
Theory
Diagrams of the vacuum filtration apparatus
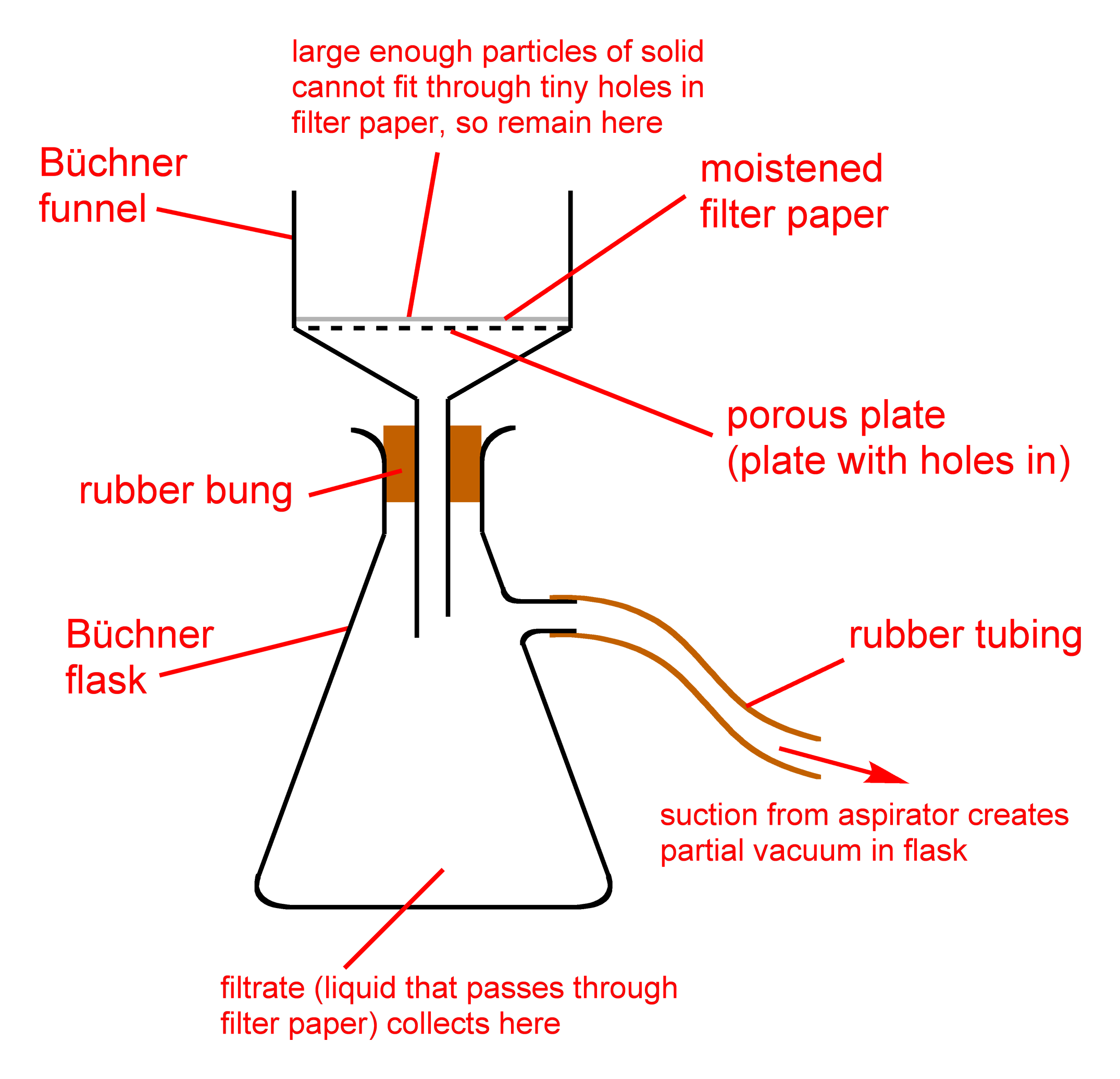
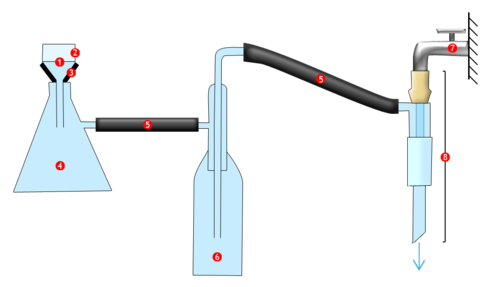
Diagram annotations: 1-Filter; 2-Büchner funnel; 3-Conic seal; 4-Büchner flask; 5-Air tube; 6-Vacuum flask; 7-Water tap; 8-Aspirator



Diagram annotations: 1-Filter; 2-Büchner funnel; 3-Conic seal; 4-Büchner flask; 5-Air tube; 6-Vacuum flask; 7-Water tap; 8-Aspirator
By flowing through the aspirator, water will suck out the air contained in the vacuum flask and the Büchner flask. There is therefore a difference in pressure between the exterior and the interior of the flasks: the contents of the Büchner funnel are sucked towards the vacuum flask. The filter, which is placed at the bottom of the Büchner funnel, separates the solids from the liquids. The solid residue, which remains at the top of the Büchner funnel, is therefore recovered more efficiently: it is much drier than it would be with a simple filtration. The rubber conical seal ensures the apparatus is hermetically closed, preventing the passage of air between the Büchner funnel and the vacuum flask. It maintains the vacuum in the apparatus and also avoids physical points of stress (glass against glass.)
The process has advantages and disadvantages in comparison to gravity filtration.
Advantages: 1) Suction filtration is much faster than gravity filtration, often taking less than one minute with good seals and a good vacuum source. 2) Suction filtration is more efficient at removing residual liquid, leading to a purer solid. This is especially important in crystallization, as the liquid may contain soluble impurities which could adsorb back onto the solid surface when the solvent evaporates.
Disadvantages: The force of suction may draw fine crystals through the filter paper pores, leading to a quantity of material that cannot be recovered from the filter paper, and possibly an additional quantity that is lost in the filtrate. This method therefore works best with large crystals. On small scales, the loss of material to the filter paper and filtrate is significant, and so other methods are recommended for microscale work.
The process has advantages and disadvantages in comparison to gravity filtration.
Advantages: 1) Suction filtration is much faster than gravity filtration, often taking less than one minute with good seals and a good vacuum source. 2) Suction filtration is more efficient at removing residual liquid, leading to a purer solid. This is especially important in crystallization, as the liquid may contain soluble impurities which could adsorb back onto the solid surface when the solvent evaporates.
Disadvantages: The force of suction may draw fine crystals through the filter paper pores, leading to a quantity of material that cannot be recovered from the filter paper, and possibly an additional quantity that is lost in the filtrate. This method therefore works best with large crystals. On small scales, the loss of material to the filter paper and filtrate is significant, and so other methods are recommended for microscale work.
As the goal of suction filtration is to fully separate a solid from its surrounding liquid, rinsing the solid is necessary if the liquid cannot easily evaporate. In the case of crystallization, the liquid may contain impurities that can reincorporate into the solid if not removed. To rinse a suction-filtered solid, the vacuum is removed and a small portion of cold solvent is poured over the solid (the "filter cake"). In the case of crystallization, the same solvent from the crystallization is used. The solid is then delicately slushed around in the solvent with a glass rod, and the vacuum is reapplied to remove the rinse solvent.
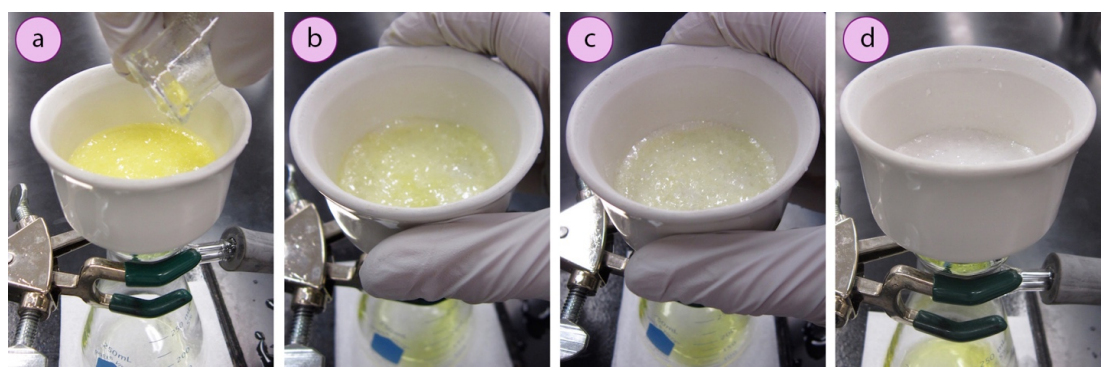
To demonstrate the importance of a rinse, Fig. 2 shows the recovery of a white solid from a yellow liquid using suction filtration. The yellow liquid seemed to be somewhat retained by the solid, as the first crystals collected had a yellow tint (Fig. 2 b). However, rinsing with a few portions of cold solvent were effective at removing the yellow liquid (Fig. 2 d), which could have been reincorporated into the solid without the rinse.
To demonstrate the importance of a rinse, Fig. 2 shows the recovery of a white solid from a yellow liquid using suction filtration. The yellow liquid seemed to be somewhat retained by the solid, as the first crystals collected had a yellow tint (Fig. 2 b). However, rinsing with a few portions of cold solvent were effective at removing the yellow liquid (Fig. 2 d), which could have been reincorporated into the solid without the rinse.
Recovery of acetanilide (white crystals) from a solution that contained yellow (methyl red) impurities. The crystals were originally tinted yellow(b), and the color faded after rinsing with cold water(c and d).
Vacuum
A water aspirator is an inexpensive attachment to a water spigot, and the nub on the aspirator connects with tubing to the vessel to be evacuated (Fig. 2 a). As water flows through the faucet and the aspirator, suction is created in the flask. A diaphragm vacuum pump can also be used.A water aspirator creates suction through the Bernoulli Principle (technically, the Venturi Effect, for liquids). Water coming from the faucet is constricted inside the aspirator (Fig.3 c). As the water flow must be the same going into the aspirator as it is going out, the water speed must increase in the constricted area in the direction of flow. A similar phenomenon can be seen in creeks and rivers, where the water flows the fastest at the narrowest portions of streams. When the water increases its velocity in the direction of the water flow, conservation of energy dictates that its velocity in perpendicular directions must decrease. The result is a lowered pressure adjacent to the fast-moving liquid. In other words, the gain in velocity of the constricted liquid is balanced by a reduction in pressure on the surrounding material (the gas).
For this reason, the speed at which the water flows through the faucet is correlated with the amount of suction experienced in the connected flask. A strong flow of water will have the fastest speeds through the aspirator and the greatest reduction in pressure.
Diaphragm vacuum pumps represent an ecological replacement for water jet pumps in laboratory use. Pumps utilise a dry compression process, avoiding waste, water or oil. With a single pump chamber ('pump head') ultimate pressures of 50 mbar are achieved. This ultimate pressure is limited due to the remaining dead volume between pumps head and diaphragm. Two pump heads in series can reach 3 mbar and three in series even 0.5 mbar. In order to rationalise the production, many manufacturers produce pump chambers and diaphragms of the same size in large quantity. This is assembled in series for lower ultimate pressure or in parallel for higher pumping speed. Membranes of Teflon® are resistant to solvents, thus suitable in chemical processes.
For this reason, the speed at which the water flows through the faucet is correlated with the amount of suction experienced in the connected flask. A strong flow of water will have the fastest speeds through the aspirator and the greatest reduction in pressure.
Diaphragm vacuum pumps represent an ecological replacement for water jet pumps in laboratory use. Pumps utilise a dry compression process, avoiding waste, water or oil. With a single pump chamber ('pump head') ultimate pressures of 50 mbar are achieved. This ultimate pressure is limited due to the remaining dead volume between pumps head and diaphragm. Two pump heads in series can reach 3 mbar and three in series even 0.5 mbar. In order to rationalise the production, many manufacturers produce pump chambers and diaphragms of the same size in large quantity. This is assembled in series for lower ultimate pressure or in parallel for higher pumping speed. Membranes of Teflon® are resistant to solvents, thus suitable in chemical processes.
Pumping speeds from 0.1 to 5 m³/h are available on the market. The higher pumping speeds are covered then by scroll pumps. Some pumps can be operated with 24V-DC-motors, enabling them to be incorporated in mobile instruments. Some have variable speed motors to reduce pumping speed (and noise) if not needed and to extend the service interval.
Application
Filtration is a unit operation that is commonly used both in laboratory and production conditions. This apparatus, adapted for laboratory work, is often used to isolate the product of synthesis of a reaction when the product is a solid in suspension. The product of synthesis is then recovered faster, and the solid is drier than in the case of a simple filtration. Other than isolating a solid, filtration is also a stage of purification: the soluble impurities in the solvent are eliminated in the filtrate (liquid).
Suction filtration widely spread in drug manufacturing. This technique is used in solid product manufacturing to obtain dry stuff. Also, it is used in pair with recrystallization technique for purificating and rinsing some substances.
Suction filtration widely spread in drug manufacturing. This technique is used in solid product manufacturing to obtain dry stuff. Also, it is used in pair with recrystallization technique for purificating and rinsing some substances.
Step-by-Step Procedures
Assemble the suction filtration flask.
1) Clamp a side-arm Erlenmeyer flask to a ring stand or latticework and attach a thick-walled rubber hose to its side arm. Connect this thick tubing to a "vacuum trap" (Fig. 4) and then to the water aspirator. It is best to not bend or strain the tubing as much as is practical, as this may cause poor suction.
A vacuum trap is necessary when connecting apparatuses to a vacuum source, as changes in pressure can cause back-suction. When using a water aspirator, back-suction might cause water from the sink to be pulled into the vacuum line and flask (ruining the filtrate), or the filtrate to be pulled into the water stream (contaminating the water supply).
2) Place a rubber sleeve (or filter adapter) and Buchner funnel atop the side-arm Erlenmeyer flask (Fig.5 a). Alternatively, use a Hirsch funnel for small scales (Fig.5 d).
3) Obtain a filter paper that will fit perfectly into the Buchner or Hirsch funnel. Filter papers are not completely flat and have a subtle arc to their shape (Fig.5 b). Place the filter paper inside the funnel concave side down (Fig.5 b and c). The paper should cover all the holes in the funnel, and with the paper arching downward (Fig.6 a), solid will be less likely to creep around the edges.
3) Obtain a filter paper that will fit perfectly into the Buchner or Hirsch funnel. Filter papers are not completely flat and have a subtle arc to their shape (Fig.5 b). Place the filter paper inside the funnel concave side down (Fig.5 b and c). The paper should cover all the holes in the funnel, and with the paper arching downward (Fig.6 a), solid will be less likely to creep around the edges.
4) Turn on the faucet connected to the water aspirator to create a strong flow of water (the degree of suction is related to the water flow). Wet the filter paper with cold solvent (using the same solvent used in crystallization, if applicable, Fig.6 b).
5) Suction should drain the liquid and hold the moist filter paper snugly over the holes in the filter. If the solvent does not drain or suction is not occurring, you may need to press down on the funnel (Fig.6 c) to create a good seal between the glass and rubber sleeve. Lack of suction may also be from a faulty aspirator or a leak in the system: to test for suction, remove the tubing from the suction flask and place your finger over the end (Fig.6 d).
5) Suction should drain the liquid and hold the moist filter paper snugly over the holes in the filter. If the solvent does not drain or suction is not occurring, you may need to press down on the funnel (Fig.6 c) to create a good seal between the glass and rubber sleeve. Lack of suction may also be from a faulty aspirator or a leak in the system: to test for suction, remove the tubing from the suction flask and place your finger over the end (Fig.6 d).
Filter and Rinse the Mixture
6) Swirl the mixture to be filtered in order to dislodge solid from the sides of the flask. If the solid is very thick, use a spatula or stirring rod to free it from the glass (Fig.7 a). In the context of crystallization, the flask will have previously been in an ice bath. Use a paper towel to dry water residue from the outside of the flask, so water does not accidentally pour onto the solid.
7) With a quick motion, swirl and dump the solid into the funnel in portions (Fig.7 b). If the solid is very thick, scoop it out of the flask onto the filter paper (Fig.7 c). It's best if the solid can be directed toward the middle of the filter paper, as solid near the edges may creep around the filter paper.
8) A small amount of chilled solvent (1-2ml for macroscale work) can be used to help rinse any residual solid from the flask into the funnel (Fig.7 d). In crystallization, it is not wise to use an excessive amount of solvent, as it will decrease the yield by dissolving small amounts of crystals. Again, press on the funnel to create a good seal and efficient drainage if necessary.
7) With a quick motion, swirl and dump the solid into the funnel in portions (Fig.7 b). If the solid is very thick, scoop it out of the flask onto the filter paper (Fig.7 c). It's best if the solid can be directed toward the middle of the filter paper, as solid near the edges may creep around the filter paper.
8) A small amount of chilled solvent (1-2ml for macroscale work) can be used to help rinse any residual solid from the flask into the funnel (Fig.7 d). In crystallization, it is not wise to use an excessive amount of solvent, as it will decrease the yield by dissolving small amounts of crystals. Again, press on the funnel to create a good seal and efficient drainage if necessary.
9) Rinse the solid on the filter paper to remove contaminants that may remain in the residual liquid.
- Break the vacuum on the flask by opening the pinch clamp at the vacuum trap (Fig.8 a) or by removing the rubber tubing on the filter flask. If adjusting the pinch clamp, you will know the system is open when there is an increase in water flow by the faucet. Then turn off the water on the aspirator. It is always important to open the system to the atmosphere before turning off the aspirator in order to prevent back-suction.
- Add 1-2ml of cold solvent (Fig.8 b). Use a glass stirring rod to break up any solid chunks and distributed the solvent to all portions of the solid (Fig.8 c), taking care to not rip or dislodge the filter paper. Reapply the vacuum to the flask, and dry the solid with suction for a few minutes.
10) After filtration is complete, again open the flask to the atmosphere by releasing the pinch clamp or opening it elsewhere, and turn off the water connected to the aspirator.
11) Transfer the solid, filter paper and all, to a pre-weighed watch glass using a spatula (Fig.8 a and b). The filter cake shouldn't be mushy, and if it is, the liquid was not adequately removed (try a different aspirator and repeat suction filtration).
12) Allow the solid to dry overnight in a desiccator, if possible before recording a final mass or melting point. The solid will flake off the filter paper more easily when completely dry (Fig.8 c).
13) If pressed for time, a solid can be quickly dried in the following ways:
12) Allow the solid to dry overnight in a desiccator, if possible before recording a final mass or melting point. The solid will flake off the filter paper more easily when completely dry (Fig.8 c).
13) If pressed for time, a solid can be quickly dried in the following ways:
- If the solid is wet with water, it can be placed in a 110 deg. oven (if the melting point is not below this temperature). If the solid is wet with organic solvent, it should never be placed in an oven as it may ignite.
- If the solid is wet with organic solvent, it can be pressed between fresh pieces of filter paper (multiple times if needed) to quickly dry them. Inevitably, some solid will be lost on the filter paper.
Attachments
Last edited:
