α-PHENYLACETOACETONITRILE from Organic Synthesis.org
Org. Synth. 1938,
18, 66
DOI: 10.15227/orgsyn.018.0066
α-PHENYLACETOACETONITRILE
[
α-Tolunitrile, α-acetyl-]
Submitted by Percy L. Julian, John J. Oliver, R. H. Kimball, Arthur B. Pike, and George D. Jefferson.
Checked by C. R. Noller and Martin Synerholm.
1. Procedure
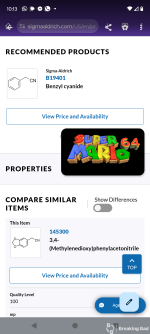
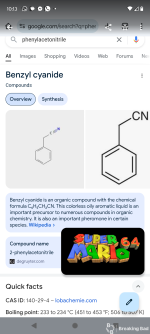
A solution of sodium ethoxide is prepared from 60 g. (2.6 gram atoms) of clean sodium and 700 cc. of absolute alcohol (Note 1) in a 2-l. round-bottomed flask equipped with a reflux condenser. To the hot solution is added a mixture of 234 g. (2 moles) of pure benzyl cyanide (Note 2) and 264 g. (3 moles) of dry ethyl acetate (Note 3). The mixture is thoroughly shaken, the condenser closed with a calcium chloride tube, and the solution heated on the steam bath for two hours before standing overnight (Note 4). The next morning the mixture is stirred with a wooden rod to break lumps, cooled in a freezing mixture to −10°, and kept at this temperature for two hours. The sodium salt is collected on a 6-in. Büchner funnel and washed four times on the funnel with 250-cc. portions of ether. The filter cake is practically colorless and corresponds to 250–275 g. of dry sodium salt, or 69–76 per cent of the calculated amount. The combined filtrates are placed in the freezing mixture until they can be worked up as indicated below.
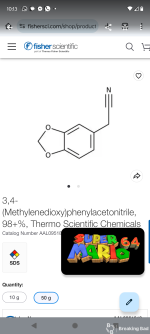
The sodium salt still wet with ether is dissolved in 1.3 l. of distilled water at room temperature, the solution cooled to 0°, and the nitrile precipitated by adding slowly, with vigorous shaking, 90 cc. of glacial acetic acid, while the temperature is kept below 10°. The precipitate is separated by suction filtration and washed four times on the funnel with 250-cc. portions of water. The moist cake weighing about 300 g. (Note 5) corresponds to 188–206 g. (59–64 per cent) of dry colorless α-phenylacetoacetonitrile, m.p. 87–89°, which is suitable for most purposes.
If it is desired to recrystallize the crude product, the moist cake is dissolved in 100 cc. of hot methyl alcohol and the solution filtered and cooled, with stirring, to −10°. The crystals are separated by suction filtration and washed once on the filter with 40 cc. of methyl alcohol cooled to −10°. When dry, the product weighs 173–191 g. (54–60 per cent) and melts at 88.5–89.5°.
The filtrates and washings from the separation of the sodium salt are placed in a 5-l. flask and diluted with ice-cold water until the flask is full; the lower layer is removed almost completely by siphoning, most of the ether removed by decantation, and the remainder separated in a separatory funnel. The aqueous layer is extracted twice in a similar manner with 500-cc. portions of ether, and the ether extracts are discarded. The ether remaining in the aqueous layer is removed under diminished pressure by drawing air through the solution for one hour with a suction pump, and the α-phenylacetoacetonitrile is precipitated by adding 60 cc. of glacial acetic acid. If an oil is thrown out, the flask is placed in an ice bath until the precipitate is crystalline. The crystals are separated by suction filtration and washed four times on the funnel with 50-cc. portions of water. When dry the tan-colored product weighs 50–55 g. and melts at 83–86°. It is dissolved in the methyl alcohol mother liquors from the crystallization of the first lot. The solution is boiled with a little Norite, filtered, and cooled to −10°. The crystals which form are collected on a filter, washed with 10 cc. of cold methyl alcohol, and dried. There is obtained 43–48 g. of pale straw-colored material, m.p. 87–89°. The product is recrystallized from 25 cc. of pure methyl alcohol and washed with 10 cc. of cold methyl alcohol; there is obtained 37–41 g., m.p. 88.5–89.5°, making a total yield of material of this purity of 210–232 g. (66–73 per cent of the theoretical amount) (Note 6) and (Note 7).
2. Notes
1. The absolute alcohol may be prepared by drying 95 per cent alcohol twice with lime, or once with lime and once with sodium according to
Note 1, Org. Syn. Coll. Vol. I, 1941, 251, or commercial absolute alcohol may be dried once with lime or sodium just before use.
2. Benzyl cyanide was prepared according to
Org. Syn. Coll. Vol. I, 1941, 107, including the purification with concentrated sulfuric acid.
3. Commercial absolute ethyl acetate was refluxed for one-half hour over phosphorus pentoxide and distilled just before use.
4. If time permits, the procedure may be continued without allowing the mixture to stand overnight. If the drying of the alcohol and ethyl acetate is begun in the morning, however, this is a convenient point at which to interrupt the procedure.
5. If used for the preparation of methyl benzyl ketone
(p. 391), the product should not be dried.
6. It does not pay to attempt to recover more pure material by concentration of the mother liquors.
7. The number of steps may be decreased by omitting the isolation of the sodium salt. If this procedure is followed, the reaction mixture, after standing overnight, is diluted in a 5-l. flask with 2 l. of water and shaken until the sodium salt dissolves. A liter of cracked ice is added and the mixture extracted with one 1-l. and two 500-cc. portions of ether. The extracted aqueous solution is freed of ether as above and precipitated with a solution of 150 cc. of glacial acetic acid in 400 cc. of water, filtered, and washed with water. The product is colored and of lower melting point than that obtained from the purified sodium salt, and must be recrystallized twice from methyl alcohol to reach a melting point of 88.5–89.5°. The total yield of material of this melting point is somewhat less than that given above.
3. Discussion
α-Phenylacetoacetonitrile has been prepared by the condensation of ethyl acetate with the sodium derivative of benzyl cyanide prepared from benzyl cyanide and sodium amide in ether,
1 and by condensation of ethyl acetate and benzyl cyanide by means of dry
2 or alcoholic
3 sodium ethoxide.
This preparation is referenced from:
References and Notes
- Bodroux, Compt, rend. 151, 234 (1910); Bull. soc. chim. (4) 7, 848 (1910).
- Walther and Schickler, J. prakt. Chem. (2) 55, 343 (1897).
- Beckh, Ber. 31, 3160 (1898); Post and Michalek, J. Am. Chem. Soc. 52, 4358 (1930).
α-PHENYLACETOACETONITRILE from Organic Synthesis.org