- Joined
- Dec 27, 2022
- Messages
- 30
- Reaction score
- 81
- Points
- 18
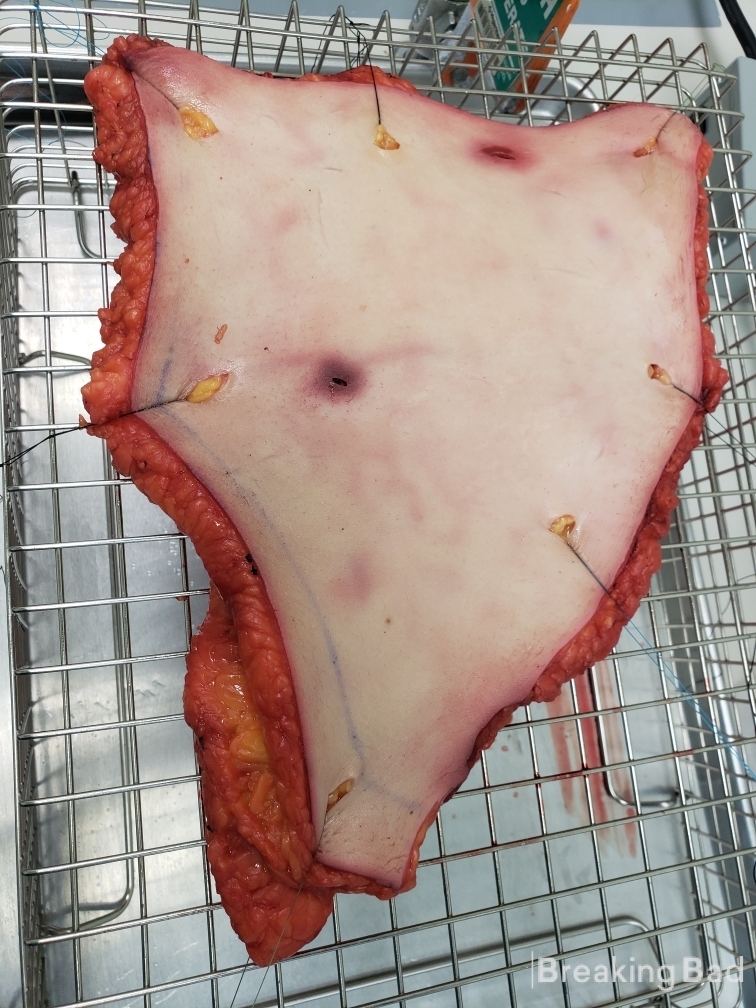
Alright guys! Time to share some knowledge with you. I will describe a synthesis I did for psilocin, complete with photos. I used the psilocin produced and developed a simple adhesive patch. In order to test the permeation of the psilocin in the patch, we utilized a diffusion apparatus I pieced together. It involves several membranes, with a 1 cm^2 patch put on top of a 1 cm^2 piece of fresh human skin @ 400 um thickness (donated from a tummy tuck surgical center). Below the skin, there is a constant flow of water that is at pH 7.4 and at body temperature. The diffusion apparatus is able to collect a 1mL fraction per hour, which is then analyzed via LC-MS. The amount of psilocin in the 1mL vial will give the flux (mg/hr/cm^2). This dictates how much psilocin passes per hour with a patch size of cm^2.
This gets deeper, as psilocin is highly unstable.The following publication, Structure Elucidation and Spectroscopic Analysis of Chromophores Produced by Oxidative Psilocin Dimerization, goes over the issue. When the dimer forms, it is undetectable via HPLC analysis and ATR-FTIR which is strange. This gives research groups false positives for their psilocin purity. I have found a way to stabilize psilocin in the patch.
Anyways, lets begin! I did every step myself, with the assistance of my partner who is a chemical engineer. This was back when I had a schedule1 and 3 DEA license not only for research, but for Manufacturing as well.
Part I (Psilocin Synthesis)
step 1: synthesis of 3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate
1a. A 2000 mL, four-necked, round-bottomed flask was equipped with an overhead stirrer, J-Kem temperature controller, a 250 mL dropping funnel, and rubber septum through which a positive pressure of dryN2 was inserted. The septum was removed and the flask was chargeds equentially with 1H-indol-4-yl acetate (5; 50.1 g, 285 mmol, 1 equiv)
nd anhyd Et2O (700 mL). The flask was resealed with the septum and is flushed with N2. The suspension was stirred for 10 min and then
cooled to 0 °C in an ice-water bath for 30 min.

1b. The dropping funnel was charged with a solution of oxalyl chloride (37.1 mL, 428 mmol, 1.5 equiv) in Et2O (60 mL). The oxalyl chloride solution was added dropwise at a rate sufficient to keep the temperature at or below 5 °C, to minimize formation of dimer and other possible by-product. As the addition progressed, a yellow slurry of 10 was formed and when the addition was completed, the mixture was stirred for 4 h.

1c. After this time, heptane (400 mL) was addedand the mixture stirred for 30 min at 0 °C. The yellow solid obtained was quickly filtered and rinsed successively with heptane (2 × 300 mL), which was quickly used in the next step. . A 2.0 M solution of dimethylamine in THF (175 mL) was added dropwise at a rate sufficient to maintaintemperature below 5 °C in order to minimize side reactions. After the
addition was complete, pyridine (46 mL) in of THF (100 mL) was add-ed dropwise and the mixture was stirred well for 60 min. Heptane (600 mL) was added and the flask contents were suction filtered via aBüchner funnel. The filtered residue was transferred into a round-bottomed flask and deionized H2O (1000 mL) was added, stirred for 30 min, and filtered via Büchner funnel. The off-white solid was tritu-rated sequentially for 40 min in EtOAc (600 mL) and heptane (400mL). The slurry was filtered via Büchner funnel and the solid wasdried in an oven at 40 °C overnight to afford 6 as a light-yellow solid;yield: 66.1 g (81%); mp 205–207 °C.



ATR-FTIR confirmed the synthesis of synthesis of 3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate. This compound can be stored at 4C for several months!
Step 2: Psilocin Synthesis
Step 2A: A 2000 mL, four-necked, round-bottomed flask was equipped with an overhead stirrer, J-Kem temperature controller, a
250 mL dropping funnel, and rubber septum through which a positive pressure of dry N2 was inserted. The septum was removed and the
flask charged sequentially with 3-[2-(dimethylamino)-2-oxoacetyl]-1H-indol-4-yl acetate (6; 31.5 g, 115 mmol) and 2-CH3-THF (1000
mL). The flask was immersed in an ice-bath at 0 °C and a solution of 2.3 M LiAlH4 in 2-CH3-THF (140 mL, 322 mmol) was added through
the 250 mL dropping funnel. The dropping funnel was rinsed with additional 2-CH3-THF (20 mL). The LiAlH4 solution was added dropwise
at a rate to maintain a temperature below 20 °C. After the addition, the ice-water bath was removed and the mixture stirred for 30 min.
.The light-yellow solution was heated to reflux (80 °C) with aheating mantle and became ivory-colored after 3 h. Accumulation of
yellow solids was observed on the sides of the round-bottomed flask



Step 2C. The heating mantle was removed, and the flask was allowed to cool to 50 °C. The flask was again
chilled to 20 °C. The reaction was quenched by sequential addition of 3 drops of aq 1 M NaOH and 3 drops of deionized H2O. The mixture
was diluted with THF (500 mL) and stirred for 20 min. The mixture was filtered via Büchner funnel and the filtrate was kept under N2.
The filter cake was quickly reslurried with 200 mL of [10% solution of (7% ammonia in MeOH) in CH2Cl2] and THF (500 mL). The filtrates
were then combined and concentrated to give a green solid. The solid was triturated with 1:1 EtOAc/heptane (50 mL), then filtered via
Büchner funnel. The dark green solid was dried in an oven at 40 °C overnight to provide dry psilocin (7) as a dark green solid; yield: 20.7
g (91%); mp 167–169 °C.
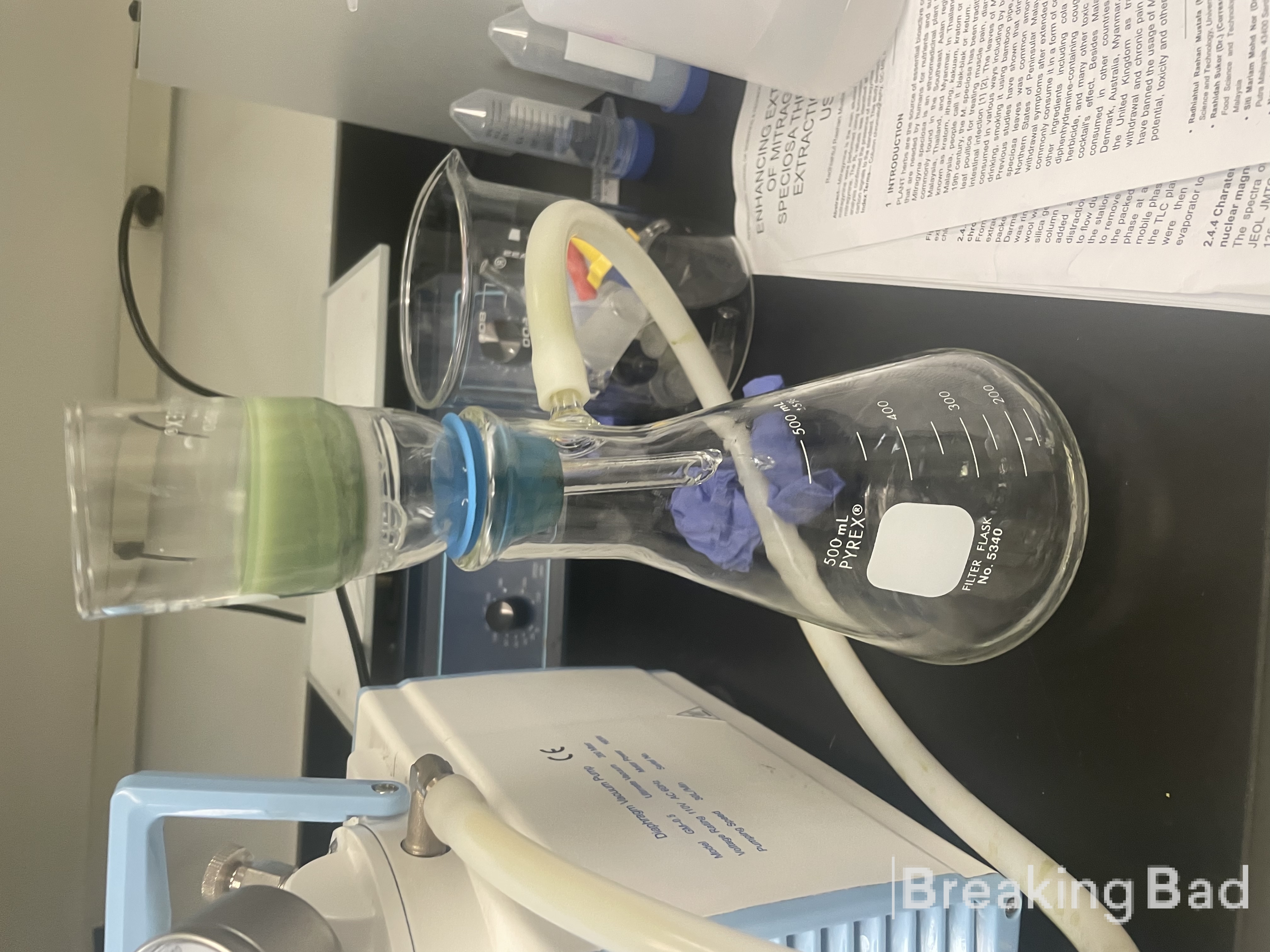


Psilocin has been synthesized.
Part II: Development of Transdermal Psilocin Patch and In-Vitro Study (CAUTION: YOU WILL SEE FRESH HUMAN FLESH, ITS GROSS
I then dissolve the psilocin into a proprietary blend of acrylate polymer (in heptane), I then cast the solution into a release line and use a casting knife to get the adhesive layer to 100 um. Once dried, I apply the backing layer and punch out a 1cm^2 patch. This patch is used for the in vitro study.
The following photo is a snapshot of the diffusion apparatus (patch on top of skin with water running below)
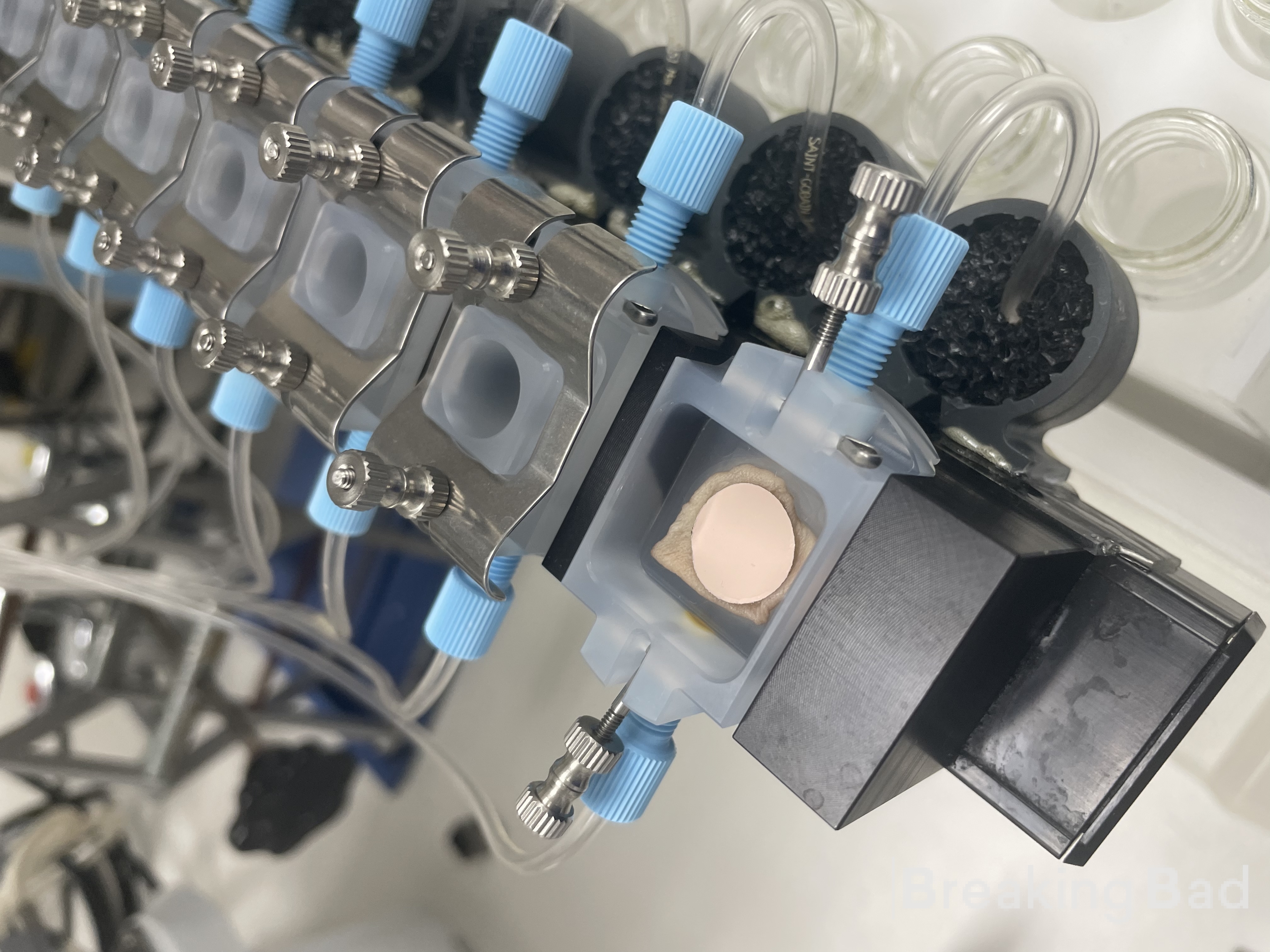
Hope you enjoyed!
This gets deeper, as psilocin is highly unstable.The following publication, Structure Elucidation and Spectroscopic Analysis of Chromophores Produced by Oxidative Psilocin Dimerization, goes over the issue. When the dimer forms, it is undetectable via HPLC analysis and ATR-FTIR which is strange. This gives research groups false positives for their psilocin purity. I have found a way to stabilize psilocin in the patch.
Anyways, lets begin! I did every step myself, with the assistance of my partner who is a chemical engineer. This was back when I had a schedule1 and 3 DEA license not only for research, but for Manufacturing as well.
Part I (Psilocin Synthesis)
step 1: synthesis of 3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate
1a. A 2000 mL, four-necked, round-bottomed flask was equipped with an overhead stirrer, J-Kem temperature controller, a 250 mL dropping funnel, and rubber septum through which a positive pressure of dryN2 was inserted. The septum was removed and the flask was chargeds equentially with 1H-indol-4-yl acetate (5; 50.1 g, 285 mmol, 1 equiv)
nd anhyd Et2O (700 mL). The flask was resealed with the septum and is flushed with N2. The suspension was stirred for 10 min and then
cooled to 0 °C in an ice-water bath for 30 min.
1b. The dropping funnel was charged with a solution of oxalyl chloride (37.1 mL, 428 mmol, 1.5 equiv) in Et2O (60 mL). The oxalyl chloride solution was added dropwise at a rate sufficient to keep the temperature at or below 5 °C, to minimize formation of dimer and other possible by-product. As the addition progressed, a yellow slurry of 10 was formed and when the addition was completed, the mixture was stirred for 4 h.
1c. After this time, heptane (400 mL) was addedand the mixture stirred for 30 min at 0 °C. The yellow solid obtained was quickly filtered and rinsed successively with heptane (2 × 300 mL), which was quickly used in the next step. . A 2.0 M solution of dimethylamine in THF (175 mL) was added dropwise at a rate sufficient to maintaintemperature below 5 °C in order to minimize side reactions. After the
addition was complete, pyridine (46 mL) in of THF (100 mL) was add-ed dropwise and the mixture was stirred well for 60 min. Heptane (600 mL) was added and the flask contents were suction filtered via aBüchner funnel. The filtered residue was transferred into a round-bottomed flask and deionized H2O (1000 mL) was added, stirred for 30 min, and filtered via Büchner funnel. The off-white solid was tritu-rated sequentially for 40 min in EtOAc (600 mL) and heptane (400mL). The slurry was filtered via Büchner funnel and the solid wasdried in an oven at 40 °C overnight to afford 6 as a light-yellow solid;yield: 66.1 g (81%); mp 205–207 °C.
ATR-FTIR confirmed the synthesis of synthesis of 3-[2-(Dimethylamino)-2-oxoacetyl]-1H-indol-4-yl Acetate. This compound can be stored at 4C for several months!
Step 2: Psilocin Synthesis
Step 2A: A 2000 mL, four-necked, round-bottomed flask was equipped with an overhead stirrer, J-Kem temperature controller, a
250 mL dropping funnel, and rubber septum through which a positive pressure of dry N2 was inserted. The septum was removed and the
flask charged sequentially with 3-[2-(dimethylamino)-2-oxoacetyl]-1H-indol-4-yl acetate (6; 31.5 g, 115 mmol) and 2-CH3-THF (1000
mL). The flask was immersed in an ice-bath at 0 °C and a solution of 2.3 M LiAlH4 in 2-CH3-THF (140 mL, 322 mmol) was added through
the 250 mL dropping funnel. The dropping funnel was rinsed with additional 2-CH3-THF (20 mL). The LiAlH4 solution was added dropwise
at a rate to maintain a temperature below 20 °C. After the addition, the ice-water bath was removed and the mixture stirred for 30 min.
.The light-yellow solution was heated to reflux (80 °C) with aheating mantle and became ivory-colored after 3 h. Accumulation of
yellow solids was observed on the sides of the round-bottomed flask
Step 2C. The heating mantle was removed, and the flask was allowed to cool to 50 °C. The flask was again
chilled to 20 °C. The reaction was quenched by sequential addition of 3 drops of aq 1 M NaOH and 3 drops of deionized H2O. The mixture
was diluted with THF (500 mL) and stirred for 20 min. The mixture was filtered via Büchner funnel and the filtrate was kept under N2.
The filter cake was quickly reslurried with 200 mL of [10% solution of (7% ammonia in MeOH) in CH2Cl2] and THF (500 mL). The filtrates
were then combined and concentrated to give a green solid. The solid was triturated with 1:1 EtOAc/heptane (50 mL), then filtered via
Büchner funnel. The dark green solid was dried in an oven at 40 °C overnight to provide dry psilocin (7) as a dark green solid; yield: 20.7
g (91%); mp 167–169 °C.
Psilocin has been synthesized.
Part II: Development of Transdermal Psilocin Patch and In-Vitro Study (CAUTION: YOU WILL SEE FRESH HUMAN FLESH, ITS GROSS
I then dissolve the psilocin into a proprietary blend of acrylate polymer (in heptane), I then cast the solution into a release line and use a casting knife to get the adhesive layer to 100 um. Once dried, I apply the backing layer and punch out a 1cm^2 patch. This patch is used for the in vitro study.
The following photo is a snapshot of the diffusion apparatus (patch on top of skin with water running below)
Hope you enjoyed!


