Brain
Expert Pharmacologist
- Joined
- Jul 6, 2021
- Messages
- 304
- Reaction score
- 332
- Points
- 63
AM-2201 (1-(5-Fluoropentyl)-[1H-indol-3-yl]-(naphthalen-1-yl)-methanone is a synthetic cannabinoid, which was initially created in 2000 as a pharmacological tool for studying endocannabinoid system. It is a powerful full agonist of cannabinoid receptors (CB1R), which causes psychoactive effects, similar to that of phytocannabinoid Δ9-tetrahydrocannabinol (THC), but its binding affinity is 40 times higher than that of THC. Similarly, binding affinity of АМ-2201 to cannabinoid receptors of type 2 (CB2R), responsible for cannabinoid-mediated peripheral effects, is 14 times higher than that of THC. Smoking is the most common way of administration, and typical doses of AM-2201 range from 250 ug to 2 mg. As a rule, AM-2201 is a part of the following substances: ‘Spice’, ‘K2’, ‘legal weed’, ‘synthetic cannabis’, ‘herbal incense‘. Also, in Germany it was found to be an additive to more than 90 different brands of ‘herbal mixtures‘, which included: Agent Orange’, ‘Atomic Bomb’, ‘Green’, ‘Jamaican Gold Extreme’, ‘Manga Xtreme’, ‘New Bonzai’ and ‘XoXo’. The Drug Abuse Warning Network (DAWN) reports about 15 thousands visits to emergency room associated with synthetic cannabinoid abuse. Metabolites of AM-2201 were identified in approximately 60% of blood samples. While heating (e. g. while smoking) AM-2201 can be transformed into JWH-018 to a minor extent. JWH-018 was one of the first synthetic cannabinoids, included in various national legislations on drug control. However, AM-2201 is not considered a direct precursor of any substance, controlled internationally. AM-2201 is a naphthoylindole, alkylated at indole nitrogen and carrying a fluorine atom at 5-position of the pentyl side chain. From the basic chemistry perspective, it can be considered relatively inert, since it is replaced by naphthoylindole motif in a recreational position C-3. Due to the aromaticity of the indole system, nitrogen does not lead to an increase in "basicity". Unlike its non-fluorinated analogues, it can transform into JWH-018 and JWH-022 at higher temperatures to a minor extent. The analytical profile of this substance is described in many different papers, and the methods used include LC-MS/MS, GC-EI-MS, LC-Q-ToF-MS, AccuTOF-DART, NMR, FTIR ATR and UV-VIS detection. It can be detected in serum, whole blood, hair, oral fluid, in urine samples the main analytical target is his main metabolites.
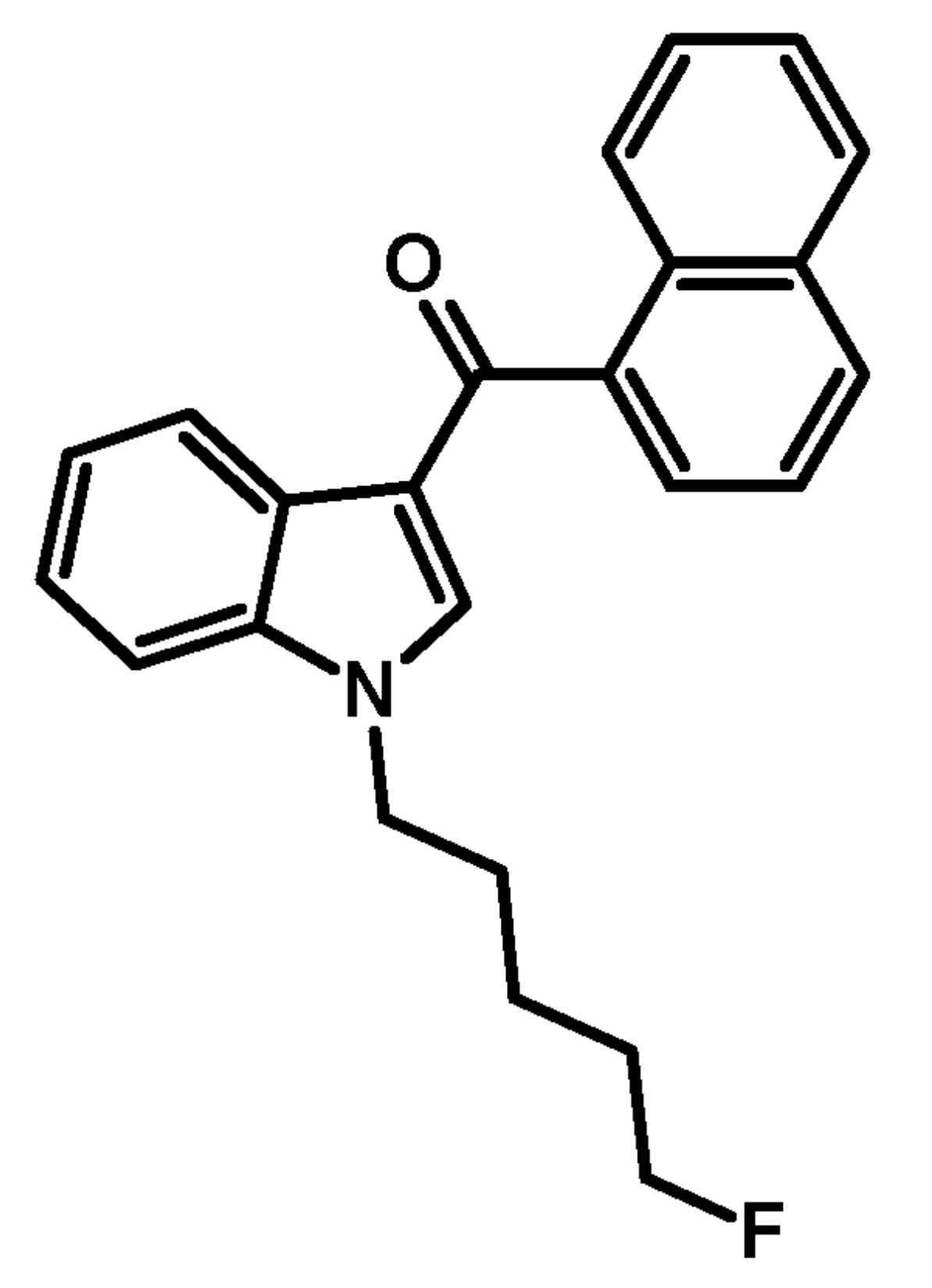
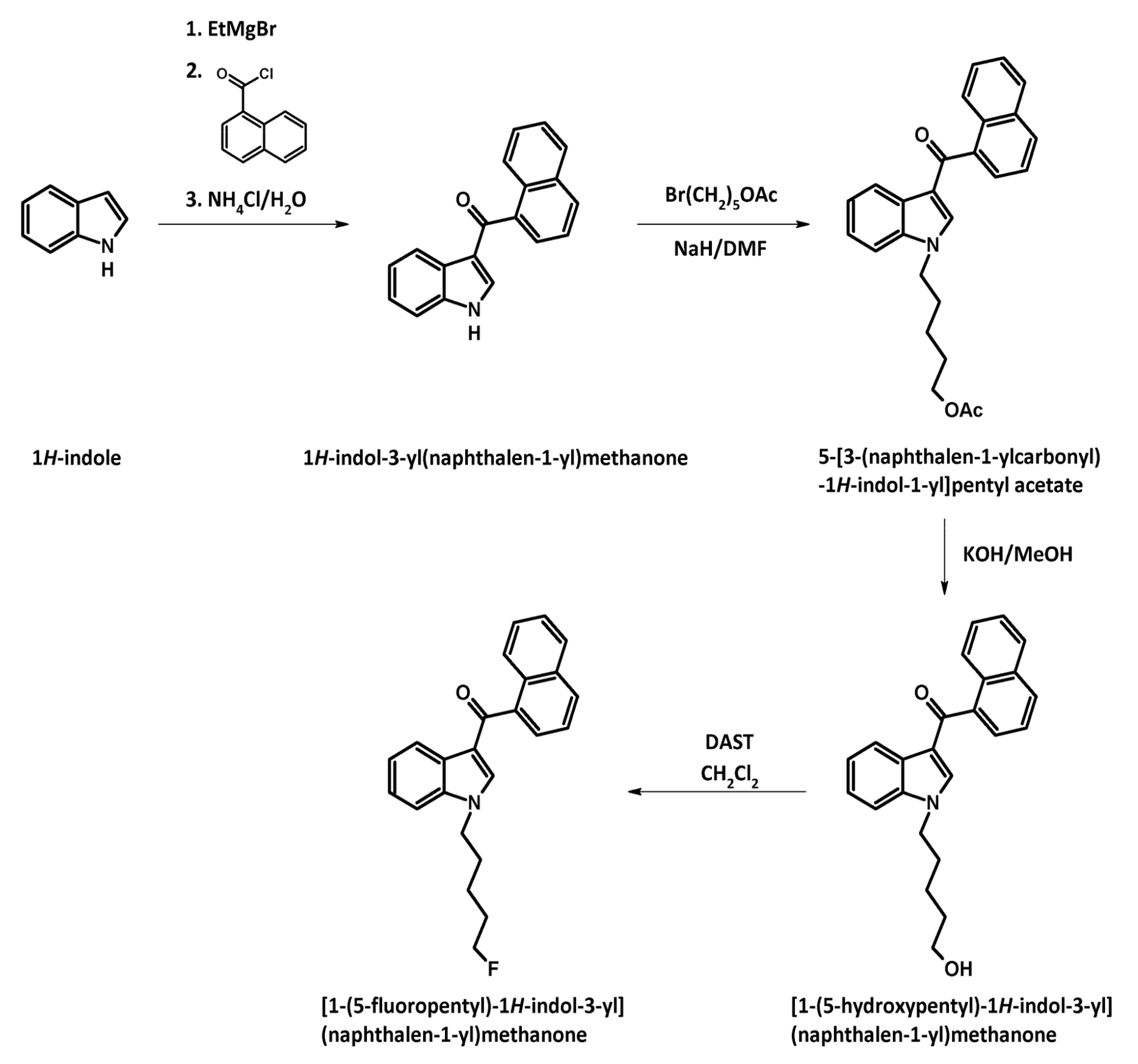
The substance itself has the appearance of white crystalline solid (in pure form), soluble in ethanol (5 mg/ml). It has a molecular formula C24H22FNO, molecular weight 359.43 g/mol, melting point 93.7 °C. The boiling point of the substance is undetermined. Synthesis of АМ-2201 was first described in 2001 by Alexandros Makriyannis and Hongfeng Deng. It starts with 1-H indole solution in the ethyl ether of acetic acid, a solution of methyl magnesium bromide in the ethyl ether of acetic acid is added. After that naphthalene-1-carbonyl chloride (prepared from naphthalene-1-carboxylic acid and thionyl chloride) is added, and, finally, an aqueous solution of ammonium chloride is added as well. Next, the resulting filtrate of 1H-indol-3- yl(naphthalen-1-yl)methanone is washed and recrystallized. This product is added to the suspension of sodium hydride in dimethylformamide (DMF), then 5-bromopentylacetate is added for N-alkylation. After cleavage of acetate by potassium hydroxide methanol solution fluorization of pentyl side chain is carried out using diethylaminosulfur trifluoride (DAST) and dichloromethane.



Pharmacokinetics and pharmacodynamics of АМ-2201.
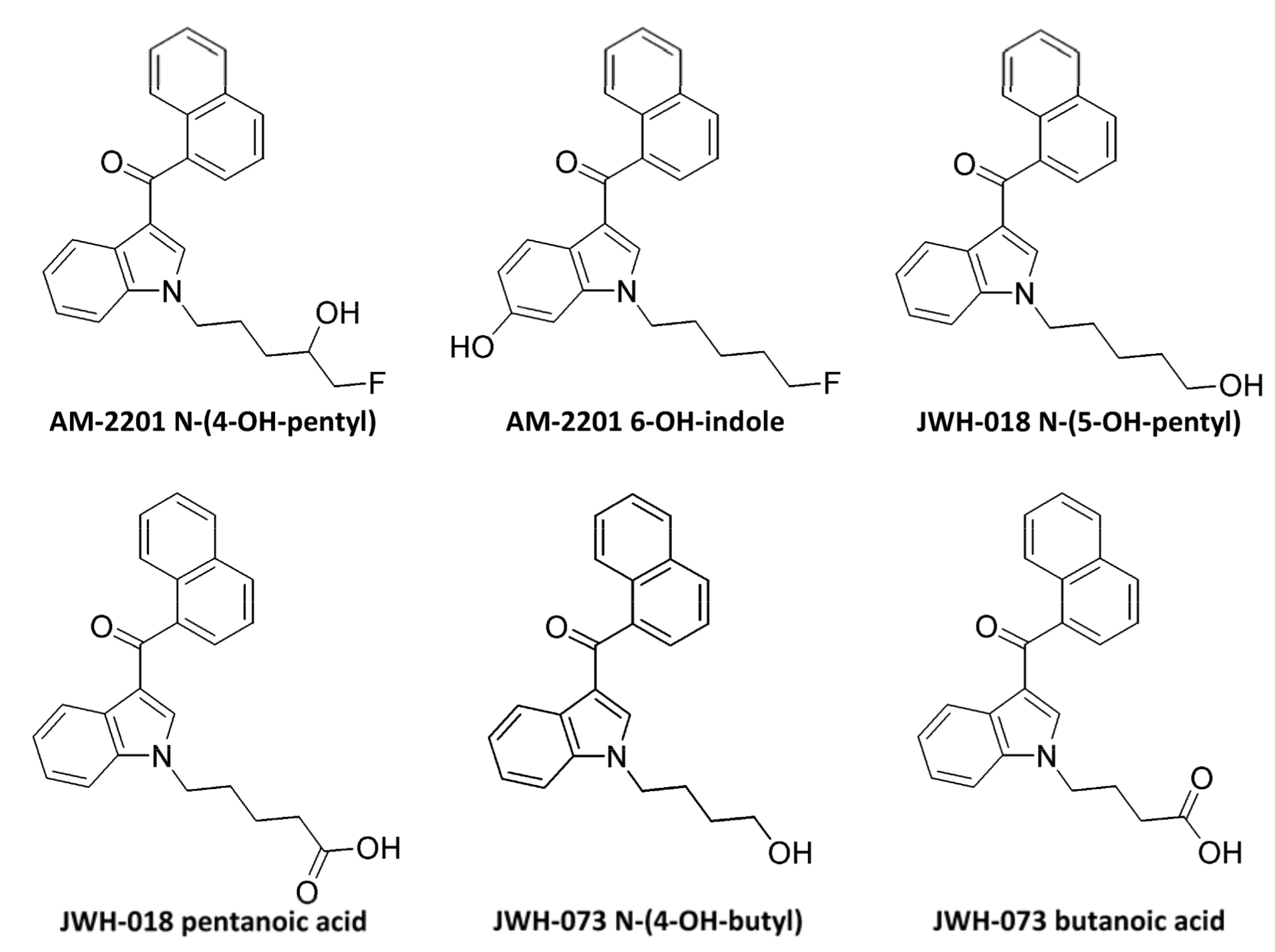
АМ-2201 is metabolized by various enzymes of the CYP450 family. In studies on human liver microsomes (HLM) and on recombinant human protein it was identified that CYP2C9 and CYP1A2 are the main enzymes, engaged in АМ-2201 oxidation, while CYP2C19, 2D6, 2E1 and 3A4 have insignificant role at this stage of metabolism. As an addition to metabolic reactions, AM-2201 undergoes enzymatic defluorination, which is presumed to be in presence of cytochrome Р450 2Е1. It is also revealed that CYP1A2, 2C9 and 2C19 mediate oxidative defluorination. In studies in vitro and in vivo, the difference between JWH-018 and А-2201 was revealed. So, JWH-018 N-(4OH-pentyl) is formed exclusively after JWH-018 absorption that is why it can be used as a diagnostic marker for differentiating between absorption of JWH-018 and AM-2201. The main metabolites include: AM-2201 N-(4-OH-pentyl), AM-2201 6-OH-indole, JWH-018 N-(5-OH-pentyl), JWH-018 pentanoic acid, JWH-073 N-(4- OH-butyl) and JWH-073 butanoic acid. Conjugation with glucuronic acid is carried out by various UDP-glucuronosyltranseferase mostly hepatic (UGT1A1, UGT1A9 and UGT2B7) and enzymes including: JWH-018 N-(5-OH- pentyl), JWH-018 pentanoic acid, JWH-073 N-(4-OH-butyl) as well as JWH-073 butanoic acid.
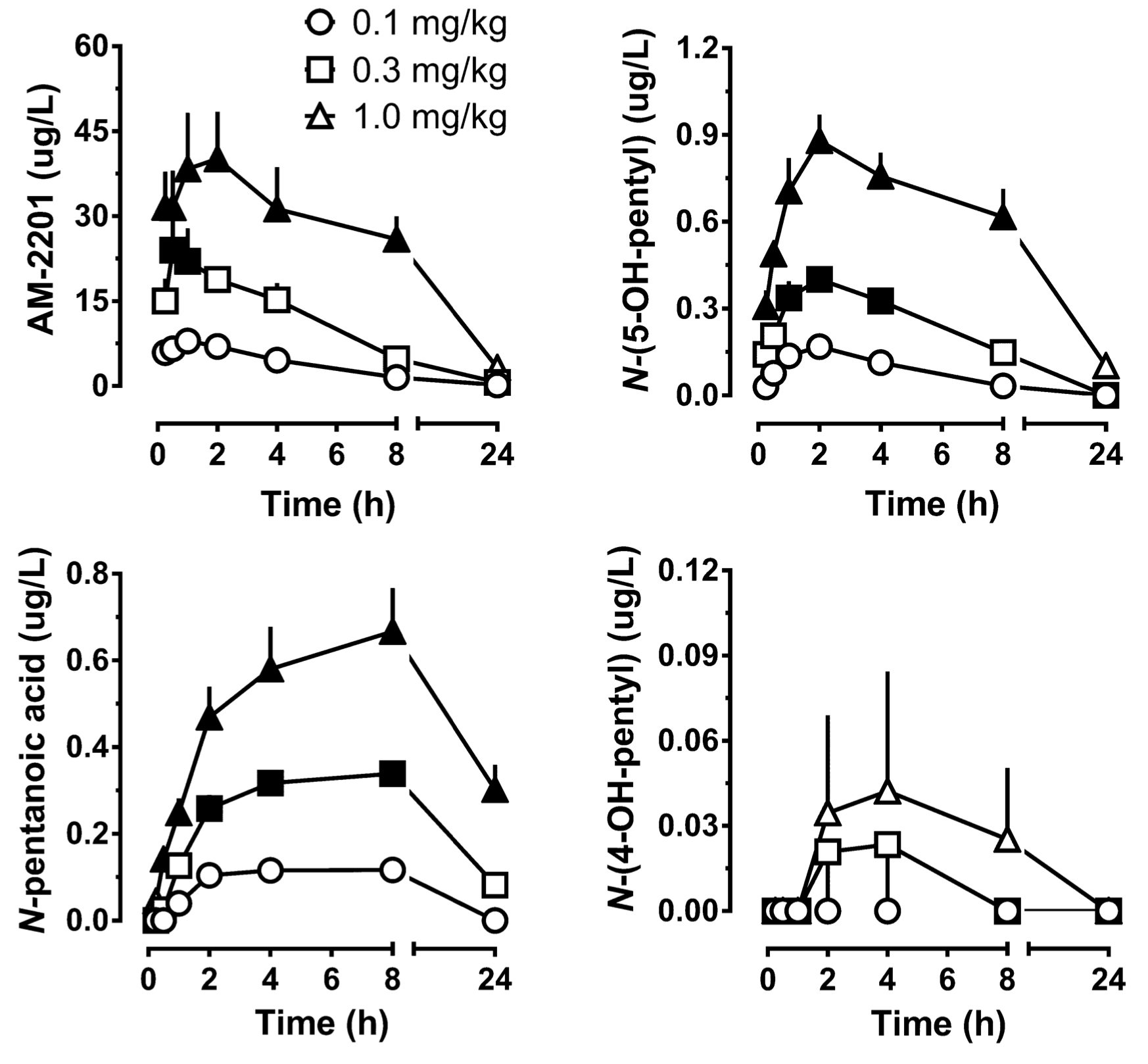
As for pharmacological constants, after administration of 5 mg of АМ-2201 peak concentration in serum with an indicator 0.56 n/ml is detected after 1 hour 35 minutes, and continues to be detectable in serum during the next five days (with detection limit of 0.8 pg/ml). This fact indicates the half-life of several days. In studies of Carlier on AM-2201 metabolism on rats time-concentration profiles were determined in plasma, which depended significantly on dose and time, whereas the concentration increased linearly as the dose increased. So, after administration of AM-2201 at a dose 0.3 mg/kg, concentration was significantly higher, than after administration of 0.1 mg/kg, while it was detectable in plasma even after 4 hours with an indicator of 3.22 μg/L. Maximum time to maximum concentration was 1.3 hours, and it didn't depend on administered dose. However, elimination half-life depended on administered dose. Time to maximum concentration of metabolite WH-018 N-(5-hydroxypentyl) was 2 hours, and the metabolite could be detected even after 24 hours. In 2013 Hutter reported the first pharmacokinetic study of AM-2201 on humans, which was based on single intake of the substance at a dose of 0.07 mg/kg. In their study, AM-2201 concentration in plasma dropped to 0.6 to <0.02 μg/L (LOQ) in 1.5-125 hours after administration. Only 4 metabolites were identified in serum: JWH-018 N-pentanoic acid, JWH-018 N-(5-hydroxypentyl), AM-2201 6’-hydroxyindole, and AM-2201 N-(4-hydroxypentyl). It is important to note, that serum concentrations of JWH-018 N-pentanoic acid exceeded that of АМ-2201 in all the samples. Maximum concentrations of JWH-018 N-(5-hydroxypentyl) and JWH-018 N-pentanoic acid were registered after 1.5 and 4.1 hours since the administration of the substance, respectively. JWH-018 N-pentanoic acid was detectable up to 57 hours after intake. Different indicators in rats and humans, presumably, are the result of interspecific differences and differences in ways of administration. So, after oral administration of AM-2201 gastrointestinal metabolism was more significant than sc-route, which is a way around this metabolic pathway. AM-2201 concentration in blood in human cases didn't exceed 5 μg/L.
Data on pharmacodynamics of the substance in vivo hasn't been revealed yet. However, it is known that it has high binding affinity to cannabinoid receptors of first type expressed as IC50 (occupation of 50% of the receptors) - 1.0 nM and to cannabinoid receptors of second type - 2.6 nM, compared to delta-9 tetrahydrocannabinol (THC) affinity - 40.7 nM for CB1- and 36.4 nM for CB2-receptors. Moreover, in studies in vitro with binding analysis of [35S] guanosine-5’-O-(3-thio)-triphosphate ([35S]GTPγS) 50% effective concentration (EC50) is determined to be 0.24 nM with full agonistic properties of the substance in terms of the receptors. Based on these data and clinical observations, it can be assumed, that AM-2201 exhibits typical effects of CB1 agonists. These effects can include sedation, cognitive dysfunction, tachycardia, postural hypotension, dry mouth, ataxia, immunosuppression and psychotropic effects. A pronounced difference from THC is formation of potentially pharmacologically active metabolites by AM-2201. While in the case of THC, only one of the main metabolites is known to have psychoactive properties, and it preserves binding affinity with cannabinoid receptors (11-OH-THC: Ki at CB1 receptor: 38.4 ± 0.8 nM). A couple of AM-2201 metabolites preserve high binding affinity to CB1 receptor with relative binding rating AM-2201 > AM-2201 N-(4-OH-pentyl) = JWH-018 N-(5-OH- pentyl) > THC > JWH-073 N-(4-OH-butyl). Glucoronidated metabolite JWH-018 N-(5-OH-pentyl) preserves its affinity for the receptor CB1 and activity as a neutral antagonist (Ki: 922 nM). There is no data regarding whether this metabolite is able to antagonize pharmacological effects of JWH-018 in vivo, or whether sufficient concentrations are formed in the site of action.
Clinical effects, doses and toxicity of АМ-2201.
Use of the substance by people with mental illness is strongly prohibited. As for potential addiction, mental dependence on the substance occurs exclusively with long-term repeated use. There is data on possibility of withdrawal syndrome occurrence, which is characterized by distorted mood, tremor in the extremities, increased anxiety, subdepressive state, spontaneous increase in heart rate and panic attacks, and the above symptoms are leveled within 2-3 months of abstinence without the pharmacological therapy.The substance itself has the appearance of white crystalline solid (in pure form), soluble in ethanol (5 mg/ml). It has a molecular formula C24H22FNO, molecular weight 359.43 g/mol, melting point 93.7 °C. The boiling point of the substance is undetermined. Synthesis of АМ-2201 was first described in 2001 by Alexandros Makriyannis and Hongfeng Deng. It starts with 1-H indole solution in the ethyl ether of acetic acid, a solution of methyl magnesium bromide in the ethyl ether of acetic acid is added. After that naphthalene-1-carbonyl chloride (prepared from naphthalene-1-carboxylic acid and thionyl chloride) is added, and, finally, an aqueous solution of ammonium chloride is added as well. Next, the resulting filtrate of 1H-indol-3- yl(naphthalen-1-yl)methanone is washed and recrystallized. This product is added to the suspension of sodium hydride in dimethylformamide (DMF), then 5-bromopentylacetate is added for N-alkylation. After cleavage of acetate by potassium hydroxide methanol solution fluorization of pentyl side chain is carried out using diethylaminosulfur trifluoride (DAST) and dichloromethane.
Pharmacokinetics and pharmacodynamics of АМ-2201.
АМ-2201 is metabolized by various enzymes of the CYP450 family. In studies on human liver microsomes (HLM) and on recombinant human protein it was identified that CYP2C9 and CYP1A2 are the main enzymes, engaged in АМ-2201 oxidation, while CYP2C19, 2D6, 2E1 and 3A4 have insignificant role at this stage of metabolism. As an addition to metabolic reactions, AM-2201 undergoes enzymatic defluorination, which is presumed to be in presence of cytochrome Р450 2Е1. It is also revealed that CYP1A2, 2C9 and 2C19 mediate oxidative defluorination. In studies in vitro and in vivo, the difference between JWH-018 and А-2201 was revealed. So, JWH-018 N-(4OH-pentyl) is formed exclusively after JWH-018 absorption that is why it can be used as a diagnostic marker for differentiating between absorption of JWH-018 and AM-2201. The main metabolites include: AM-2201 N-(4-OH-pentyl), AM-2201 6-OH-indole, JWH-018 N-(5-OH-pentyl), JWH-018 pentanoic acid, JWH-073 N-(4- OH-butyl) and JWH-073 butanoic acid. Conjugation with glucuronic acid is carried out by various UDP-glucuronosyltranseferase mostly hepatic (UGT1A1, UGT1A9 and UGT2B7) and enzymes including: JWH-018 N-(5-OH- pentyl), JWH-018 pentanoic acid, JWH-073 N-(4-OH-butyl) as well as JWH-073 butanoic acid.
As for pharmacological constants, after administration of 5 mg of АМ-2201 peak concentration in serum with an indicator 0.56 n/ml is detected after 1 hour 35 minutes, and continues to be detectable in serum during the next five days (with detection limit of 0.8 pg/ml). This fact indicates the half-life of several days. In studies of Carlier on AM-2201 metabolism on rats time-concentration profiles were determined in plasma, which depended significantly on dose and time, whereas the concentration increased linearly as the dose increased. So, after administration of AM-2201 at a dose 0.3 mg/kg, concentration was significantly higher, than after administration of 0.1 mg/kg, while it was detectable in plasma even after 4 hours with an indicator of 3.22 μg/L. Maximum time to maximum concentration was 1.3 hours, and it didn't depend on administered dose. However, elimination half-life depended on administered dose. Time to maximum concentration of metabolite WH-018 N-(5-hydroxypentyl) was 2 hours, and the metabolite could be detected even after 24 hours. In 2013 Hutter reported the first pharmacokinetic study of AM-2201 on humans, which was based on single intake of the substance at a dose of 0.07 mg/kg. In their study, AM-2201 concentration in plasma dropped to 0.6 to <0.02 μg/L (LOQ) in 1.5-125 hours after administration. Only 4 metabolites were identified in serum: JWH-018 N-pentanoic acid, JWH-018 N-(5-hydroxypentyl), AM-2201 6’-hydroxyindole, and AM-2201 N-(4-hydroxypentyl). It is important to note, that serum concentrations of JWH-018 N-pentanoic acid exceeded that of АМ-2201 in all the samples. Maximum concentrations of JWH-018 N-(5-hydroxypentyl) and JWH-018 N-pentanoic acid were registered after 1.5 and 4.1 hours since the administration of the substance, respectively. JWH-018 N-pentanoic acid was detectable up to 57 hours after intake. Different indicators in rats and humans, presumably, are the result of interspecific differences and differences in ways of administration. So, after oral administration of AM-2201 gastrointestinal metabolism was more significant than sc-route, which is a way around this metabolic pathway. AM-2201 concentration in blood in human cases didn't exceed 5 μg/L.
Data on pharmacodynamics of the substance in vivo hasn't been revealed yet. However, it is known that it has high binding affinity to cannabinoid receptors of first type expressed as IC50 (occupation of 50% of the receptors) - 1.0 nM and to cannabinoid receptors of second type - 2.6 nM, compared to delta-9 tetrahydrocannabinol (THC) affinity - 40.7 nM for CB1- and 36.4 nM for CB2-receptors. Moreover, in studies in vitro with binding analysis of [35S] guanosine-5’-O-(3-thio)-triphosphate ([35S]GTPγS) 50% effective concentration (EC50) is determined to be 0.24 nM with full agonistic properties of the substance in terms of the receptors. Based on these data and clinical observations, it can be assumed, that AM-2201 exhibits typical effects of CB1 agonists. These effects can include sedation, cognitive dysfunction, tachycardia, postural hypotension, dry mouth, ataxia, immunosuppression and psychotropic effects. A pronounced difference from THC is formation of potentially pharmacologically active metabolites by AM-2201. While in the case of THC, only one of the main metabolites is known to have psychoactive properties, and it preserves binding affinity with cannabinoid receptors (11-OH-THC: Ki at CB1 receptor: 38.4 ± 0.8 nM). A couple of AM-2201 metabolites preserve high binding affinity to CB1 receptor with relative binding rating AM-2201 > AM-2201 N-(4-OH-pentyl) = JWH-018 N-(5-OH- pentyl) > THC > JWH-073 N-(4-OH-butyl). Glucoronidated metabolite JWH-018 N-(5-OH-pentyl) preserves its affinity for the receptor CB1 and activity as a neutral antagonist (Ki: 922 nM). There is no data regarding whether this metabolite is able to antagonize pharmacological effects of JWH-018 in vivo, or whether sufficient concentrations are formed in the site of action.
Clinical effects, doses and toxicity of АМ-2201.
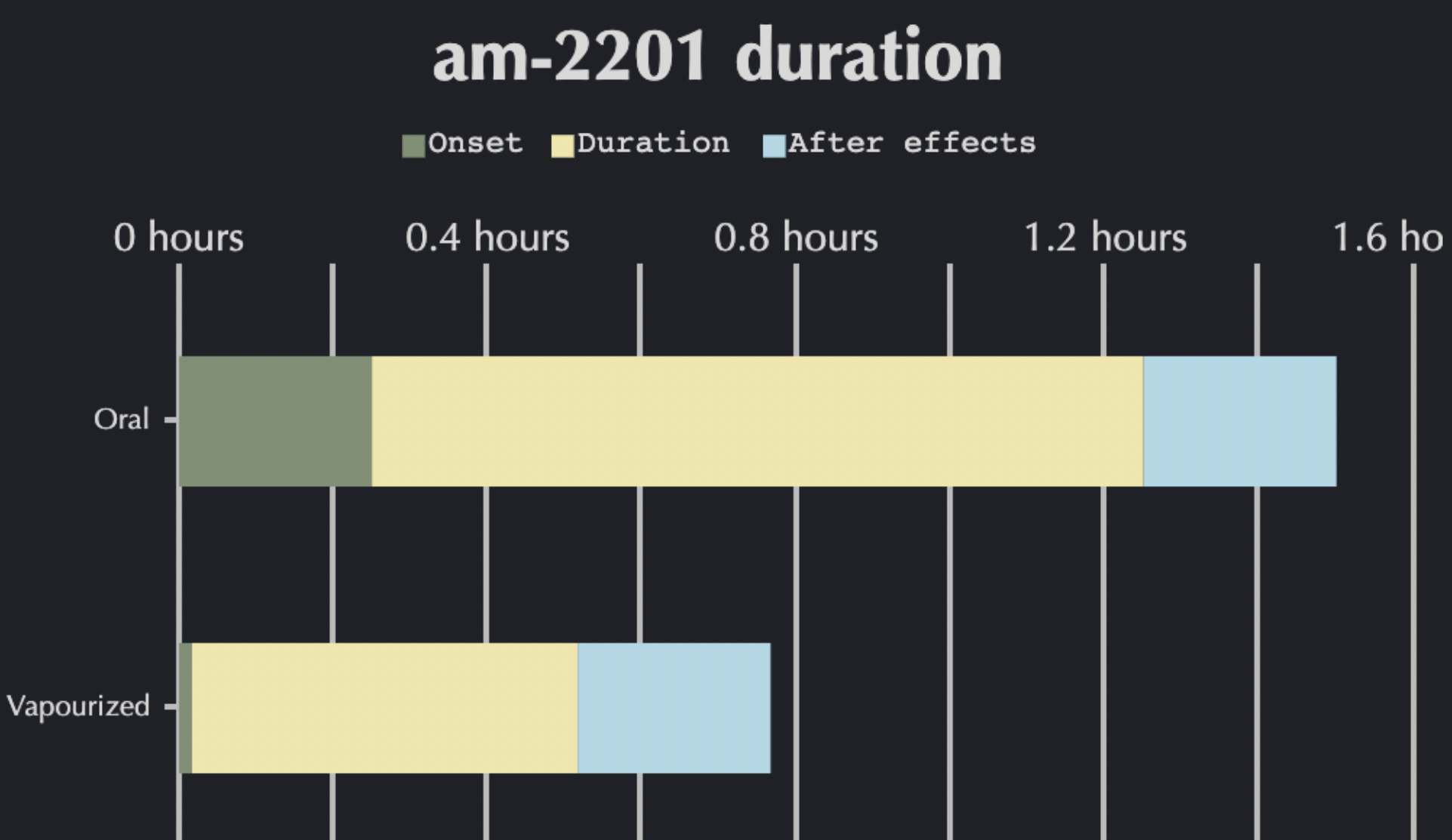
Share of АМ-2201 in various ‘herbal mixtures’ are heterogeneous and may vary (according to different sources) from 1 to 300 mg/g. Average indicator ranges within 30 mg/g. Considering the fact that the most common method of AM-2201 administration is smoking, identification of exact doses in a mixture is impossible. However, by analyzing the research data, a dose gradation of pure substance can be made in terms of THC and CBD percentage in the substance. So, the starting minimal dose (including the data of to EMCDDA) of АМ-2201, which is associated with significant and perceptible clinical effects, is 3-4 μg/kg. Medium doses range from 8 to 17 μg/kg. Any dose exceeding 20 μg/kg is high, and is considered to imply the highest risk of developing pronounced side effects. Considering a potentially high risk of developing side effects, it is not recommended to use AM-2201 in high doses due to loss of control over physical and mental state. When administered through inhalation, the onset of effects is within 5 minutes and the peak of effects is at 30-50 minutes. Effects can last for 60-180 minutes, post-effects period lasts up to 5 hours depending on the dose.
Positive desirable effects of the substance include the very same effects that occur due to THC use: euphoria, moderate relaxation, cheerful behavior, some illusions, and so on. However, considering the fact that AM-2201 is a synthetic cannabinoid (and due to its pharmacodynamic specificity), it has more pronounced side effects, which include: swaying (shaky gait), impaired coordination, increased blood pressure (or a pronounced decrease) and pulse, aphasia, convulsions, aggressive behavior, slow movements, redness of the conjunctiva, hallucinations, drowsiness, sopor or coma, trembling of the eyelids, bleeding from the gums, mydriasis, disorientation, anxiety and paranoia, depression (in post-effects), depersonalization / derealization syndrome occurs rarely. Also, negative effects include tachycardia, agitation, hallucinations, hypertension, a slight increase in blood glucose, hypokalemia, vomiting, chest pain, convulsions, myoclonia, severe anxiety leading to panic attacks and acute psychosis.
AM-2201 toxicity has been studied only on primary neural cells of the forebrain and showed induction of cytotoxicity depending on concentration. During pre-incubation with a selective antagonist of CB1, cytotoxicity of АМ-2201 (30 μM) was depressed, which indicates an important role of CB1 receptors in cytotoxicity induction of this line of cells, not in other mechanisms. Moreover, АМ-2201 cytotoxicity occurs through apoptosis and is mediated by caspases, which indicates a strong neurotoxic effect. Due to the lipophilic nature of this substance, it cannot be excluded that higher concentrations take place in the deep sections (through the accumulation effect) or in the epithelial cells of the digestive tract (which are directly exposed to smoke or pure substance). Neurotoxicity of AM-2201 is proved by the fact that endocannabinoid system is already formed in the developing central nervous system from the moment of conception and also by the fact that cannabimimetic -55,212-2, interfering in endocannabinoid system, causes anencephaly and neurobehavioral disorders in offspring. It can also be assumed that, considering physic-chemical properties of the substance, it can pass to fetal tissues through the placenta. The most common clinical withdrawal symptoms are: anxiety, unstable mood, crying outbursts, feeling of inner emptiness, shortness of breath, hyperacusis, somatic pain, hyperventilation, intense sweating, motor and internal anxiety, insomnia, cough, impaired concentration.
Positive desirable effects of the substance include the very same effects that occur due to THC use: euphoria, moderate relaxation, cheerful behavior, some illusions, and so on. However, considering the fact that AM-2201 is a synthetic cannabinoid (and due to its pharmacodynamic specificity), it has more pronounced side effects, which include: swaying (shaky gait), impaired coordination, increased blood pressure (or a pronounced decrease) and pulse, aphasia, convulsions, aggressive behavior, slow movements, redness of the conjunctiva, hallucinations, drowsiness, sopor or coma, trembling of the eyelids, bleeding from the gums, mydriasis, disorientation, anxiety and paranoia, depression (in post-effects), depersonalization / derealization syndrome occurs rarely. Also, negative effects include tachycardia, agitation, hallucinations, hypertension, a slight increase in blood glucose, hypokalemia, vomiting, chest pain, convulsions, myoclonia, severe anxiety leading to panic attacks and acute psychosis.
AM-2201 toxicity has been studied only on primary neural cells of the forebrain and showed induction of cytotoxicity depending on concentration. During pre-incubation with a selective antagonist of CB1, cytotoxicity of АМ-2201 (30 μM) was depressed, which indicates an important role of CB1 receptors in cytotoxicity induction of this line of cells, not in other mechanisms. Moreover, АМ-2201 cytotoxicity occurs through apoptosis and is mediated by caspases, which indicates a strong neurotoxic effect. Due to the lipophilic nature of this substance, it cannot be excluded that higher concentrations take place in the deep sections (through the accumulation effect) or in the epithelial cells of the digestive tract (which are directly exposed to smoke or pure substance). Neurotoxicity of AM-2201 is proved by the fact that endocannabinoid system is already formed in the developing central nervous system from the moment of conception and also by the fact that cannabimimetic -55,212-2, interfering in endocannabinoid system, causes anencephaly and neurobehavioral disorders in offspring. It can also be assumed that, considering physic-chemical properties of the substance, it can pass to fetal tissues through the placenta. The most common clinical withdrawal symptoms are: anxiety, unstable mood, crying outbursts, feeling of inner emptiness, shortness of breath, hyperacusis, somatic pain, hyperventilation, intense sweating, motor and internal anxiety, insomnia, cough, impaired concentration.
Last edited by a moderator:
