G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,596
- Solutions
- 3
- Reaction score
- 2,623
- Points
- 113
- Deals
- 1
Introduction
Diethyl ether, or CH3CH2-O-CH2CH3 (Et2O), is a great solvent for many reactions, but is extremely flammable. Professional chemists are well-informed of the hazards presented in using ether, but the layman is less likely to be aware of these dangers. Diethyl Ether vapors in dry air can form explosive peroxides. In other words, even in a spark/flame free environment, explosions can still happen when ether vapour is encountered. For this reason, should have some way of removing vapours from the vicinity (a fume hood would be a fine example) and one should not use ether on days with extremely low humidity. Because diethyl ether is so flammable, and prone to ignition, this procedure should be carried out using a hotplater/stirrer designed for use in flammable environments. Such a heater/stirrer does not produce a contact spark when the hotplate is turned on, and generally employs a brushless AC motor for the stirrer because DC motors with brushes generally produce small sparks which could ignite any stray vapours.
Diethyl ether is prepared from ethanol (a.k.a. grain alcohol, ethyl alcohol, drinking alcohol) by heating it with concentrated sulfuric acid. The reaction proceeds through an intermediary, "Ethyl sulfuric acid", as do most reactions of this type. You can easily scale this synthesis by enlarging reaction flask and number of substances proportionally.
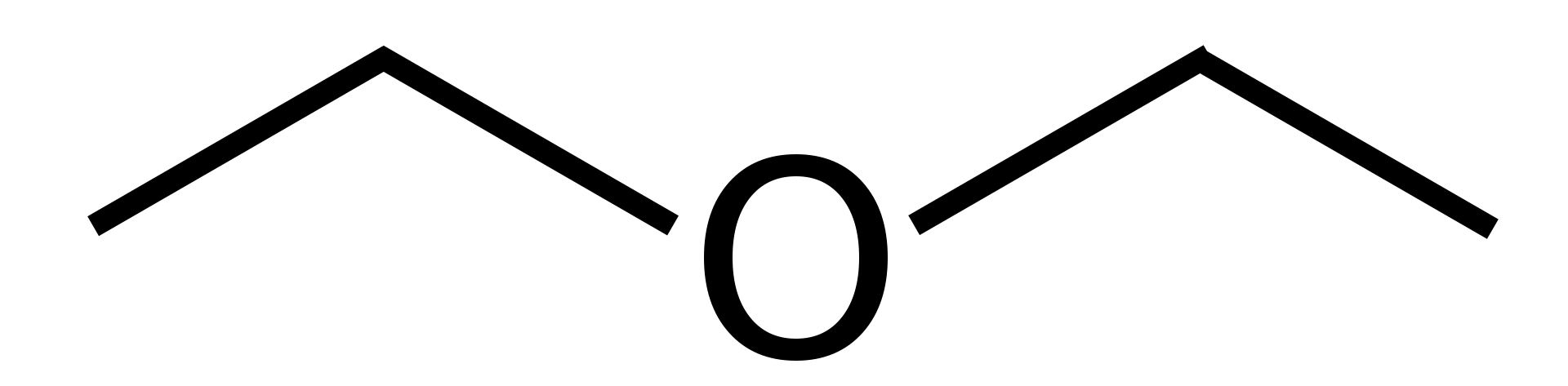
Appearance: colorless liquid with dry, rum-like, sweetish odor;Diethyl ether is prepared from ethanol (a.k.a. grain alcohol, ethyl alcohol, drinking alcohol) by heating it with concentrated sulfuric acid. The reaction proceeds through an intermediary, "Ethyl sulfuric acid", as do most reactions of this type. You can easily scale this synthesis by enlarging reaction flask and number of substances proportionally.
Boiling Point: 34.65 °C/760 mm Hg;
Melting Point: -116.3 °C;
Molecular Weight: 74.123 g/mol;
Density: 0.7134 g/ml (20 °C).
Equipment and glassware:
Fractional distillation setup;0.5 L Three neck round-bottom flask
Magnetic stirrer;
Oil and ice water bathes;
0.5 L x2 Drip funnel;
Laboratory grade thermometer (0 °C to 200 °C) with flask adapter;
pH indicator strips;
Retort stand and clamp for securing apparatus;
Laboratory scale (1 — 200 g is suitable);
100 ml x2 and 200 ml x2 Erlenmeyer flasks with caps;
200 ml x2; 100 ml x2 Beakers;
500 ml or 100 ml Measuring cylinder;
Boiling stones.
Reagents:
123.9 ml (97 g, 2 mol) Ethanol 95%;109 ml (200.16 g, 2 mol) Sulfuric acid (98%);
50 g Sodium hydroxide (NaOH);
50 g Sodium chloride (NaCl);
15 g Calcium chloride (CaCl2);
~1.5 L Distilled water;
~20 g Metallic sodium (Na)
Manipulations
1. Though this is not the most pleasant process, diethyl ether may be produced by the condensation of ethanol. To do this, assemble a typical fractional distillation setup with a vigreux column and a three neck flask. Don't forget to drop a stirrer magnet into the flask before clamping everything up, and you will be heating this on an oil bath (no flames allowed when Ether is around, you know). The vigreux column goes in the central neck, an addition funnel in one side beck, and a thermometer goes in the other side neck.
2. Add 2x moles (where the x is a multiplier, 1 = 2 moles, 1.5 = 3 moles, etc) of the azeotrope of ethanol (i.e. 95% ethanol) to the flask. Add 2x moles of concentrated (98 %) sulfuric acid to the ethanol slowly (it will heat because of the water) via drip funnel. Turn on the stirrer, turn on the heat, and bring the flask up to 130 °C. Make sure your condenser is well supplied with cold water, and continue heating till the contents of the reaction flask reach 135 °C or so.
3. Once distillation commences, slowly add up to another 2x moles of ethanol through the addition funnel at a rate equal to the drops coming from the condenser. 2 moles of alcohol (123.9 g) should take 1 hour with a decent vigreux column. A shorter column (or, gasp, no column at all) will require slower distillation (and if you don't use a column, you will have to do some extensive washing of the product with saltwater).
4. Dump the receiver flask contents into a large beaker or bowl and swirl with 10% sodium hydroxide (NaOH aq) solution until the pH is neutral. Pour this mixture into a separatory funnel to separate the Ether from the aqueous hydroxide, and wash twice more with equal volumes of half-saturated sodium chloride (NaCl) solution (~18 g/100 ml of water at r.t.). Let the last wash solution + ether rest in the flask until everything has settled, then carefully drain off the wash, and pour the Ether out of the top into a round bottom flask. Add 15 g of calcium chloride (CaCl2) (damp rid) for every mole of ether, drop in stirrer magnet and stir for 2 hours.
5. Distill the Ether from the Calcium Chloride by heating on a bath (oil or water) at no higher than 45 °C Collect distillate that comes over in the range of 31-36 °C.
Anhydrous Ethyl Ether. This is for those formulas calling for dry, pure, or anhydrous ether. The ether product from above is dried over thin slices of metallic sodium (metallic sodium wire works well also) for 24 hours. Then the ether is distilled on a water bath, over fresh (fresh means a different batch than what you used to dry with) metallic sodium.
Note: Ether develops explosive peroxides upon sitting for any length of time, even if just purchased from a supply house. Therefore, before handling ether, which has been stored, shake with ferrous sulphate or with lead peroxide. To keep peroxides from forming in fresh ether; add several sections of copper or iron wire to the dark container and store in a cool place.
Purification
4. Dump the receiver flask contents into a large beaker or bowl and swirl with 10% sodium hydroxide (NaOH aq) solution until the pH is neutral. Pour this mixture into a separatory funnel to separate the Ether from the aqueous hydroxide, and wash twice more with equal volumes of half-saturated sodium chloride (NaCl) solution (~18 g/100 ml of water at r.t.). Let the last wash solution + ether rest in the flask until everything has settled, then carefully drain off the wash, and pour the Ether out of the top into a round bottom flask. Add 15 g of calcium chloride (CaCl2) (damp rid) for every mole of ether, drop in stirrer magnet and stir for 2 hours.
5. Distill the Ether from the Calcium Chloride by heating on a bath (oil or water) at no higher than 45 °C Collect distillate that comes over in the range of 31-36 °C.
Anhydrous Ethyl Ether. This is for those formulas calling for dry, pure, or anhydrous ether. The ether product from above is dried over thin slices of metallic sodium (metallic sodium wire works well also) for 24 hours. Then the ether is distilled on a water bath, over fresh (fresh means a different batch than what you used to dry with) metallic sodium.
Note: Ether develops explosive peroxides upon sitting for any length of time, even if just purchased from a supply house. Therefore, before handling ether, which has been stored, shake with ferrous sulphate or with lead peroxide. To keep peroxides from forming in fresh ether; add several sections of copper or iron wire to the dark container and store in a cool place.
Purification
Purifying and Drying Diethyl Ether For Grignard Reactions Using Potassium Hydroxide and Sodium
Purifying and Drying Diethyl Ether For Grignard Reactions Using Potassium Hydroxide and Sodium
Diethyl ether and heptane extraction from Starter Fluid
Diethyl ether and heptane extraction from Starter Fluid
Last edited:
