Introduction
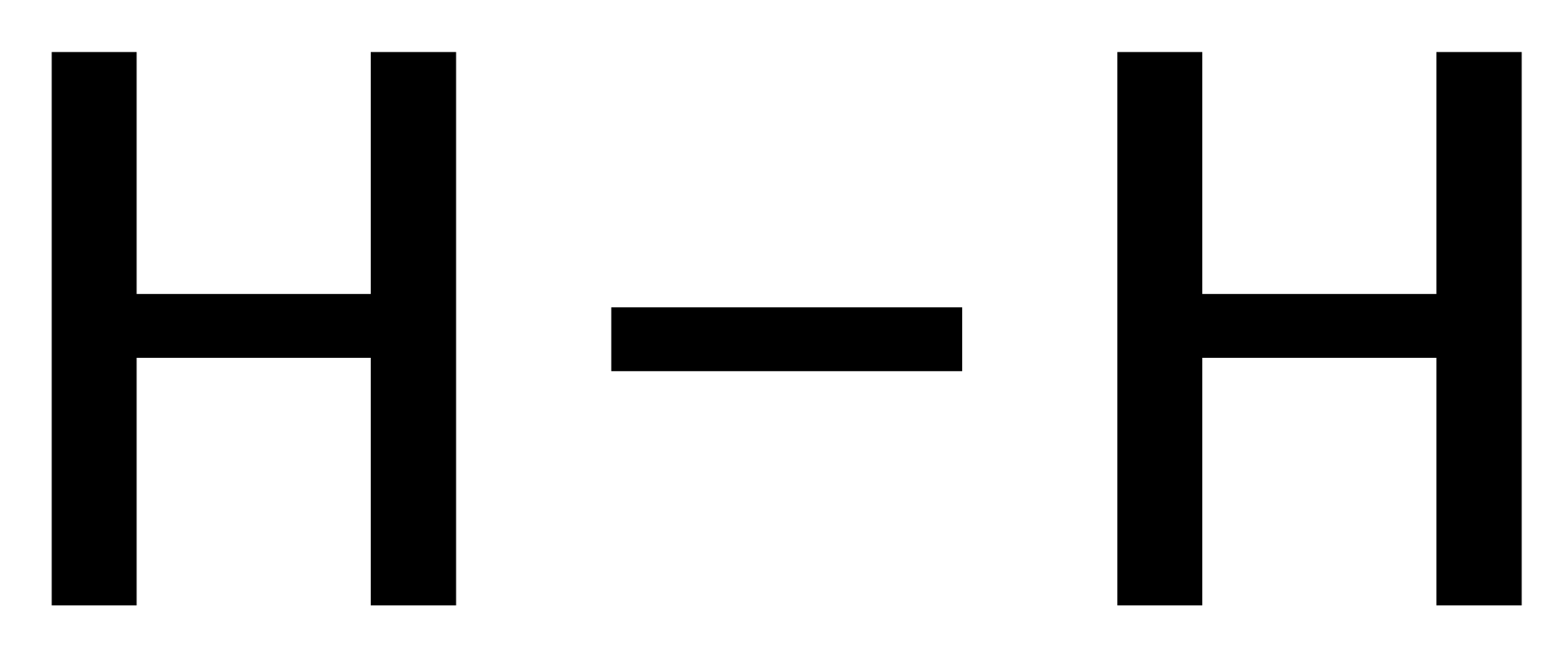
General Format: Metal + Dilute Acid → Salt of Metal & Acid + Hydrogen
With Hydrochloric Acid: Zn + 2HCl → ZnCl2 + H2
With Sulphuric Acid: Zn + H2SO4 → ZnSO4 + H2
Finally, the hydrogen gas can be collected by the downward displacement of water.
Method for producing hydrogen using Kipp's apparatus

Gaseous hydrogen used in laboratory practice as a reducing agent. Some reduction reaction in drug manufacturing using Hydrogenation procedure such as reduction of P2NP to Amphetamine, Dezocine (Dalgan), Levorphanol and Racemorphan syntheses. Hydrogen gas can substitute hard-to-reach reduction reagents such as NaBH4, NaBH4, LiAlH4 and etc in some reactions. Pay attention to safety measures during work with hydrogen gas because this gas extremely flammable and explosive.
There is short video of hydrogenation in small scale with Pd/C catalyst shown as example for underground chemist.
There is short video of hydrogenation in small scale with Pd/C catalyst shown as example for underground chemist.
Characteristics and Uses of Hydrogen Gas
Hydrogen gas is a colourless gas which does not have any distinct odour. This gas is sparingly soluble in water. The solubility of this gas in water is not affected too much by any changes in temperature. Some uses of hydrogen gas are listed below.
Procedure
The laboratory preparation of hydrogen gas usually involves the action of dilute sulphuric acid or dilute hydrochloric acid on zinc granules. Granulated zinc is ideal for the preparation of hydrogen gas in chemical laboratories because it usually contains a small amount of copper, which has the ability to act as a catalyst to the associated chemical reaction and, therefore, increase the rate of the chemical reaction without actually participating in it. An experimental procedure for the laboratory preparation of hydrogen gas is provided below.Procedure for the Laboratory Preparation of Hydrogen Gas
Step 1: Take a few grams of zinc granules and place them in a 500 mL flask.
Step 2: With the help of a thistle funnel, add dilute hydrochloric acid to the zinc granules. If hydrochloric acid isn’t available, dilute sulphuric acid can be used as an alternative.
Step 3: Hydrogen gas will be automatically collected with the help of a delivery tube via the downward displacement of water. This can be explained by the fact that hydrogen gas is lighter than water.
The setup for the laboratory preparation of hydrogen gas is illustrated below.
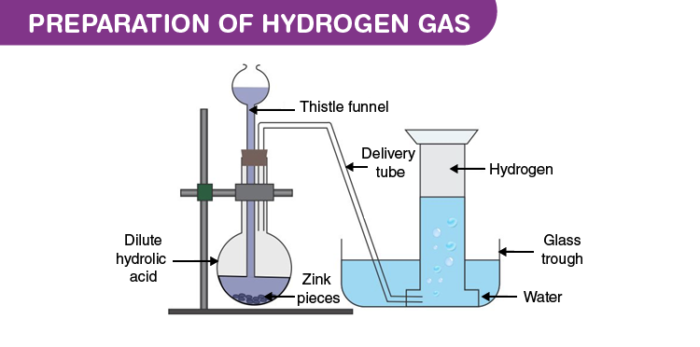
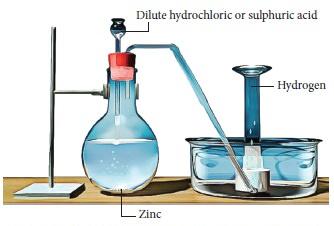
The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below.General Format: Metal + Dilute Acid → Salt of Metal & Acid + Hydrogen
With Hydrochloric Acid: Zn + 2HCl → ZnCl2 + H2
With Sulphuric Acid: Zn + H2SO4 → ZnSO4 + H2
Finally, the hydrogen gas can be collected by the downward displacement of water.
Precautions to be Taken While Preparing Hydrogen Gas in the Laboratory
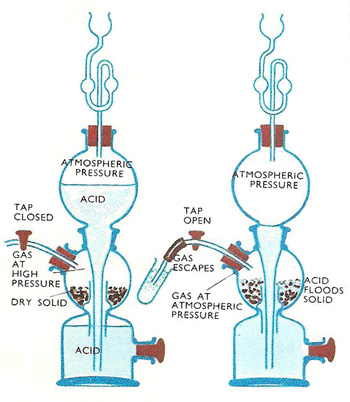
Before collecting the hydrogen gas with the help of the apparatus, precautions must be taken in order to ensure that all the air inside the apparatus has been displaced. This is because hydrogen gas reacts explosively with air.Method for producing hydrogen using Kipp's apparatus

Kipp's apparatus is an elaborate piece of laboratory glassware used, until quite recently, for preparing and storing small volumes of certain gases, notably hydrogen. It is named after its inventor, the Dutch pharmacist Petrus Johannes Kipp (1808–1864). Kipp's apparatus, also known as a Kipp generator, has now been superseded for the production of hydrogen by the use of acid and metal that convert to hydrogen gas.
In any chemical laboratory where hydrogen reduction is carried out, there needs to be a supply of hydrogen gas which can be turned on and off at will. Usually, when a gas is made in the laboratory, the apparatus has to be set up each time the gas is needed. Furthermore, there is no way of switching the supply on and off. For hydrogen and some gases, Kipp's apparatus overcame this problem. The same apparatus can also be used for supplying carbon dioxide or hydrogen sulfide on tap.
Although a regular supply of other gases may also be needed, these are the only three common gases for which the Kipp's apparatus can be used. This is because to produce other gases, heating is required. This is out of the question in the Kipp's apparatus because it would shatter on heating. The gas flow is controlled by making gas only when a cold liquid is in contact with lumps of solid. Hydrogen, carbon dioxide, and hydrogen sulfide are all made in this way. When the liquid is drained away from the solid, the supply stops. No heating is needed to make them. They are made by the action of cold acids on pieces of solid. Broken sticks of ferrous sulfide are used for making hydrogen sulfide, marble chippings for carbon dioxide, and zinc granules for hydrogen.
The Kipp's apparatus is made of thick glassware and usually stands about 1 ft 6 in (about 0.5 m) high. Other sizes are also made. Basically, it consists of three glass bulbs connected one above another. The solid needed to make the gas is placed in the central bulb by lifting off the top bulb and the glass tube fitted to it. A ground glass fitting connects this top section to the lower part. A glass fitting stops the solid from falling down into the bottom bulb. The gas exit tube leaves from the central bulb. On it is a tap for regulating the supply of gas. The gas tap is opened and acid is poured in via the funnel at the top. The uppermost section acts as a funnel to feed the lower section. There is no direct path from the top to the middle bulb. Sufficient acid is poured in to fill the bottom section and flood the solid in the center bulb. The gas tap is closed. Gas is produced, and the pressure builds up inside the bulb, forcing the acid down into the bottom bulb and up into the top one. When the liquid is forced out of the center bulb, the generation of gas stops. The apparatus is now set up, ready for use.
In any chemical laboratory where hydrogen reduction is carried out, there needs to be a supply of hydrogen gas which can be turned on and off at will. Usually, when a gas is made in the laboratory, the apparatus has to be set up each time the gas is needed. Furthermore, there is no way of switching the supply on and off. For hydrogen and some gases, Kipp's apparatus overcame this problem. The same apparatus can also be used for supplying carbon dioxide or hydrogen sulfide on tap.
Although a regular supply of other gases may also be needed, these are the only three common gases for which the Kipp's apparatus can be used. This is because to produce other gases, heating is required. This is out of the question in the Kipp's apparatus because it would shatter on heating. The gas flow is controlled by making gas only when a cold liquid is in contact with lumps of solid. Hydrogen, carbon dioxide, and hydrogen sulfide are all made in this way. When the liquid is drained away from the solid, the supply stops. No heating is needed to make them. They are made by the action of cold acids on pieces of solid. Broken sticks of ferrous sulfide are used for making hydrogen sulfide, marble chippings for carbon dioxide, and zinc granules for hydrogen.
The Kipp's apparatus is made of thick glassware and usually stands about 1 ft 6 in (about 0.5 m) high. Other sizes are also made. Basically, it consists of three glass bulbs connected one above another. The solid needed to make the gas is placed in the central bulb by lifting off the top bulb and the glass tube fitted to it. A ground glass fitting connects this top section to the lower part. A glass fitting stops the solid from falling down into the bottom bulb. The gas exit tube leaves from the central bulb. On it is a tap for regulating the supply of gas. The gas tap is opened and acid is poured in via the funnel at the top. The uppermost section acts as a funnel to feed the lower section. There is no direct path from the top to the middle bulb. Sufficient acid is poured in to fill the bottom section and flood the solid in the center bulb. The gas tap is closed. Gas is produced, and the pressure builds up inside the bulb, forcing the acid down into the bottom bulb and up into the top one. When the liquid is forced out of the center bulb, the generation of gas stops. The apparatus is now set up, ready for use.
When gas is needed, the tap is turned on. The gas pressure in the center bulb is released. There is no extra pressure to hold the acid in the top bulb, so it drops down to completely fill the bottom bulb and once more flood the solid. When the gas tap is turned off, as the gas can no longer escape, the pressure again builds up, forcing the liquid back into the top bulb or reservoir. The build up of pressure ceases when all drops of acid left clinging to the solid have been used up.
In time, the acid grows weaker, and the solid is used up. The chemicals require renewing. The acid is drained out by removing the bung from the lower bulb, after which the remaining solid can be taken out. This should be done in a fume cupboard to prevent the breathing of poisonous fumes. Because of its poisonous qualities and unpleasant smell of bad eggs, it is advisable to always keep a hydrogen sulfide Kipp's apparatus in the fume cupboard.
In time, the acid grows weaker, and the solid is used up. The chemicals require renewing. The acid is drained out by removing the bung from the lower bulb, after which the remaining solid can be taken out. This should be done in a fume cupboard to prevent the breathing of poisonous fumes. Because of its poisonous qualities and unpleasant smell of bad eggs, it is advisable to always keep a hydrogen sulfide Kipp's apparatus in the fume cupboard.
Last edited:

