Introduction.
Meticulously dry or oxygen-free conditions are sometimes necessary when using reagents that react with water or oxygen in the air. To safely and effectively use these reagents, glassware should be oven or flame dried, then the air displaced with a dry, inert gas (often nitrogen or argon). This creates an "inert atmosphere" inside an apparatus, one that will not react with the reagents.
Setting up a Reaction.
Every reaction requires the addition of several reagents, often in a proscribed order. When performing an air-sensitive reaction, adding the reagents without introducing air or moisture into the system takes care and skill. How to add each reagent depends upon the nature of the reaction. Reactions can be air-sensitive in terms of reagents, such as n-BuLi or Grignard reagents, or it may be that only the product of the reaction is air-sensitive, for example, an organometallic complex.
If the reaction necessary to carry out in an inert atmosphere, first fill an empty apparatus with an inert gas. Then add (quickly!) Solvents and reagents, purge the flask with an inert gas, cooling (if it is necessary). The reaction is carried out under a minimum buffer overpressure of an inert gas. The excess pressure is created by a short (several mm) column of inert liquid poured into a liquid shutter at the outlet of this device. The synthesis is carried out by carefully following the procedure, maintaining the cooling (heating) mode and the rate of addition of the reagents. In Fig.1 depicts an apparatus for carrying out synthesis in an inert atmosphere with cooling with temperature control. The resulting mixture is processed in strict accordance with the procedure at the end of the synthesis. The simplest method for isolating the target compound is filtration. In some cases, a pure product is possible to isolate by distillation (steam distillation) directly from the reaction mixture. In other cases, the first stage in the reaction mixtures processing is the quenching of reactive reagents and intermediates (water, solutions of acids or bases), neutralization of acid or alkaline catalysts, separation of insoluble compounds by filtration, extraction of the product from the inorganic or aqueous phase, and its concentration. In this case, extraction is mainly used.
If the reaction necessary to carry out in an inert atmosphere, first fill an empty apparatus with an inert gas. Then add (quickly!) Solvents and reagents, purge the flask with an inert gas, cooling (if it is necessary). The reaction is carried out under a minimum buffer overpressure of an inert gas. The excess pressure is created by a short (several mm) column of inert liquid poured into a liquid shutter at the outlet of this device. The synthesis is carried out by carefully following the procedure, maintaining the cooling (heating) mode and the rate of addition of the reagents. In Fig.1 depicts an apparatus for carrying out synthesis in an inert atmosphere with cooling with temperature control. The resulting mixture is processed in strict accordance with the procedure at the end of the synthesis. The simplest method for isolating the target compound is filtration. In some cases, a pure product is possible to isolate by distillation (steam distillation) directly from the reaction mixture. In other cases, the first stage in the reaction mixtures processing is the quenching of reactive reagents and intermediates (water, solutions of acids or bases), neutralization of acid or alkaline catalysts, separation of insoluble compounds by filtration, extraction of the product from the inorganic or aqueous phase, and its concentration. In this case, extraction is mainly used.
Fig.1
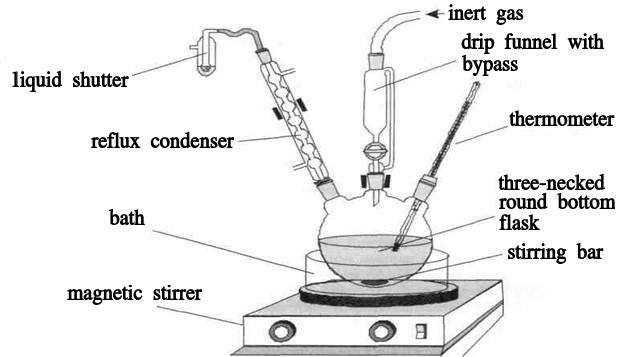
Experimental Set-Ups.
Reactions are set up in the same way as non-air-sensitive reactions, but with either a three-necked flask or Schlenk flask instead of the traditional round-bottomed flask. Schlenk flasks have a tap to connect the apparatus to the inert gas line and control the access to inert gas, while a three-necked flask requires an additional adaptor for this purpose. The simplest way to set up a reaction is to assemble all the glassware first and then apply to evacuate and refill cycle to ensure an inert atmosphere before adding the reagents. However, it is possible to fill each piece with equipment separately and assemble the kit with a positive flow of inert gas coming from each piece of glassware, i.e., there is sufficient gas flow into the apparatus that a steady stream is emitted when a stopper is removed, for example. This is more time-consuming, but can be a useful technique if you need to add or remove a piece of glassware midway through an experiment (see below). Typical reactions set-ups are shown in Fig.2.
.
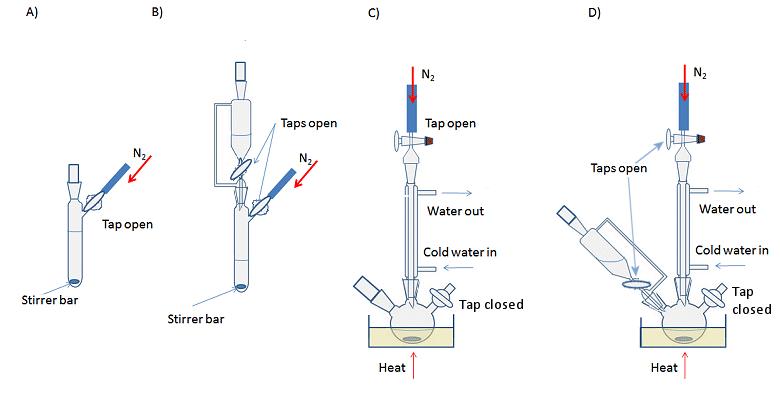
Another difference compared with air-stable chemistry is that all the ground-glass joints must be greased to ensure an airtight seal and prevent contamination by O2, for example. In organic chemistry labs, it is often recommended to leave joints ungreased, as grease can enter the reaction mixture and contaminate the reaction and spectra. For similar reasons, joints should not be over-greased when doing air-sensitive chemistry. A fine layer of grease applied evenly is better than a thick layer that seeps out of the top and bottom of the joint. To get a thin layer, apply two stripes of grease, on opposite sides of the male joint of any glassware, insert into the neck or female joint and rotate the two parts gently to evenly distribute the grease (Fig.3). There should be a clear, continuous film between the surfaces of the joint.
Occasionally, it will be necessary to add or remove a piece of glassware, such as an addition funnel, during the course of the reaction. To add a piece of glassware, ensure that it is clean and dry of any cleaning solvent or water and all joints are greased. Attach it to the inert gas line and fill it. Ensure there are two positive flows of inert gas, i.e., the gas is flowing out of the vessel: one flow coming from the reaction vessel and the other from the glassware to be attached. Once there is a steady stream of inert gas flowing from each part of the apparatus, the stopper from the reaction vessel can be removed, and the glassware added in its place. To remove glassware during a reaction, simply ensure there is a positive flow of inert gas coming from the reaction vessel before removing the glassware and replacing it with a stopper or cap.
Greasing Joints.
Ground glass joints are manufactured to fit quite well with one another, and yet they are not perfectly airtight. In some situations (e.g. when using reduced pressure inside an apparatus or inert atmosphere), grease must be applied to each joint to ensure a good seal. Grease is also used whenever the joint may be in contact with a highly basic solution, as basic solutions can form sodium silicates and etch glass.
Grease can be applied with a syringe full of grease (Fig.4 a), wood splint, or toothpick. Grease should be lightly applied in portions around the male joint, closer to the glass end than the end which will be in contact with reagents (Fig.4 a). If grease is allowed near the end which will contact the reagents, there is a possibility the reagent will dissolve the grease and become contaminated. The female joint should then be connected, and the joints twisted to spread the grease in a thin layer. The joint should become transparent all the way around the joint, but to a depth of only one-third to one-half of the joint (Fig.4 b). If the entire joint becomes transparent or if grease is seen spilling out of the joint, too much grease has been using (Fig.4 c). Excess grease should be wiped off with a KimWipe (one is used in Fig. 5).
Grease can be applied with a syringe full of grease (Fig.4 a), wood splint, or toothpick. Grease should be lightly applied in portions around the male joint, closer to the glass end than the end which will be in contact with reagents (Fig.4 a). If grease is allowed near the end which will contact the reagents, there is a possibility the reagent will dissolve the grease and become contaminated. The female joint should then be connected, and the joints twisted to spread the grease in a thin layer. The joint should become transparent all the way around the joint, but to a depth of only one-third to one-half of the joint (Fig.4 b). If the entire joint becomes transparent or if grease is seen spilling out of the joint, too much grease has been using (Fig.4 c). Excess grease should be wiped off with a KimWipe (one is used in Fig. 5).
To clean grease from a joint after a process is complete, wipe of the majority of the grease using a paper towel or KimWipe. Then wet a KimWipe with some hydrocarbon solvent and rub the moistened KimWipe onto the joint to dissolve the grease (Fig. 5). Hydrocarbon solvents (e.g. hexanes) work much better than acetone to dissolve residual grease.
.

Adding Air-Stable Solids at the Beginning of a Reaction.
A solid can be added at any stage of the reaction, however, if you can, add all the solids to the reaction flask first as manipulation becomes more difficult once a liquid has been added. If the solid is air-stable, it can be added directly to the flask at the beginning of the reaction, as described below.
To add air-stable solids at the beginning of a reaction:
To add air-stable solids at the beginning of a reaction:
- Weigh the solid and add it to a clean and dry Schlenk flask or three-necked round-bottomed flask as appropriate for the reaction to be performed.
- Stopper flask with a greased, ground-glass stopper.
- Attach the flask to the inert gas line.
- Open the flask to the line carefully, taking care that the solid/powder is not blown out. This would result in less reagent being in the reaction than originally calculated.
- Ensure the flask is filled with inert gas and open to the line so that there is a positive flow of gas before adding anything else.
Adding Air-Sensitive Solid at the Beginning of a Reaction.
The best way to add an air-sensitive solid as the first reagent is to use a balance in a glovebox to weigh out the solid and put it in the flask, all under an inert atmosphere. Many air-sensitive solids are stored in a glovebox on a permanent basis.
Adding Solids Mid-Way Through a Reaction.
To add an air-stable solid:
- Weigh the required amount of solid into a vial or weighing boat.
- Ensure there is a positive flow of inert gas into the reaction vessel. Make sure the vessel is open to the inert gas line and that the bubbler has 2–3 bubbles per second flowing through it.
- Remove the stopper of the reaction vessel.
- Place a powder funnel in the open neck. This will prevent the solid sticking to the greased neck as it is added. If you don’t have a powder funnel, a piece of paper bent into a cone-shape will work just as well – just ensure the end is lower than the base of the neck.
- Carefully pour the solid into the reaction via the funnel. The positive flow of inert gas out of the flask may cause some solid, particularly if it is a powder, to be lost if the hole is blocked, so add the solid slowly and in small portions if you are adding a lot of powder. Take care to avoid inhaling any airborne powder. Alternatively, hold the funnel so that the joint is inside the neck of the flask, but there is a gap between the joint and the neck. This will give the inert gas room to escape without blowing through the funnel and blowing the powder everywhere. The problem with this method is that you are more likely to get powder stuck to the greased neck of the flask.
- Remove the funnel and replace the stopper once all the solid is added.
To add an air-sensitive solid
Air-sensitive solids should be added in a glovebox as described above. Some reactions, however, are not amenable to moving the entire apparatus or the reaction vessel into the glovebox, for example, variable temperature additions. If this is the case, there are two possible approaches: A) using a solid addition tube and B) dissolving the solid to add as a solution.
A) Solid Addition Tube
A solid addition tube is a simple piece of glassware similar to a test tube but with a bend in it and with a ground-glass joint suitable for placing inside the neck of a reaction vessel (Fig. 13). Some have a Schlenk or Young's tap to allow control of the internal atmosphere and gas flow. The air-sensitive compound is placed in the tube in a glovebox (see above) and the tube is sealed with a cap or stopper depending on the type of joint (Fig. 6, A). Once outside the glovebox, the tube is attached to the inert gas line using the standard procedure. The cap is the removed under a positive flow of inert gas and the tube is inserted into the neck of the flask also under a positive flow of inert gas (Fig. 6, B). The tube can then be rotated or gently tapped to encourage the solid to fall into the reaction vessel. If you are using a solid addition tube without a tap, a secondary stream of inert gas can be introduced using the set-up shown in Fig. 6, C. This provides an inert-gas blanket during the opening and attaching of the tube to the flask. This technique is also useful if you need to open an ampule of air-sensitive solid.
A) Solid Addition Tube
A solid addition tube is a simple piece of glassware similar to a test tube but with a bend in it and with a ground-glass joint suitable for placing inside the neck of a reaction vessel (Fig. 13). Some have a Schlenk or Young's tap to allow control of the internal atmosphere and gas flow. The air-sensitive compound is placed in the tube in a glovebox (see above) and the tube is sealed with a cap or stopper depending on the type of joint (Fig. 6, A). Once outside the glovebox, the tube is attached to the inert gas line using the standard procedure. The cap is the removed under a positive flow of inert gas and the tube is inserted into the neck of the flask also under a positive flow of inert gas (Fig. 6, B). The tube can then be rotated or gently tapped to encourage the solid to fall into the reaction vessel. If you are using a solid addition tube without a tap, a secondary stream of inert gas can be introduced using the set-up shown in Fig. 6, C. This provides an inert-gas blanket during the opening and attaching of the tube to the flask. This technique is also useful if you need to open an ampule of air-sensitive solid.
B) Adding an Air-Sensitive Solid as a Solution.
Perhaps the simplest and most effective way of adding an air-sensitive solid is to weigh it into a separate, clean, dry Schlenk flask under an inert atmosphere and dissolve it in a suitable solvent. The resulting solution can then be added to the reaction mixture via a cannula (see below).
Perhaps the simplest and most effective way of adding an air-sensitive solid is to weigh it into a separate, clean, dry Schlenk flask under an inert atmosphere and dissolve it in a suitable solvent. The resulting solution can then be added to the reaction mixture via a cannula (see below).
Adding Solvents and Liquids.
Liquids can be transferred to or from vessels attached to an inert gas line and under an inert atmosphere easily with either a syringe or a double-ended stainless-steel needle, called a cannula. Which you use will depend on the quantity and reactivity of the liquid to be transferred and, to a certain extent, the design of the container the liquid is being transferred from. As a general rule, up to 50 ml can be transferred with a syringe, while larger quantities are usually transferred with a cannula.
Syringe techniques.
Many air-sensitive chemicals are supplied as solutions in nitrogen-filled bottles, which are sealed by a septum, and small volumes (up to 25 ml) of these solutions are best transferred to the apparatus using glass syringes. Similarly, air-sensitive liquids can be added to the reaction using a syringe.
*Note: When removing air-sensitive reagents from nitrogenfilled bottles, you must replace the volume of liquid removed with inert gas (nitrogen) from a gas cylinder or balloon, via a needle, otherwise air (water, oxygen and carbon dioxide) will be pulled into the bottle as a result of the vacuum you have created.
Syringes.
Glass, gas-tight syringes with a Luer lock fitting are the most versatile type of syringe, and they come in a range of sizes. The Luer lock enables the stainless-steel needle to be locked in place on the end of the syringe so that there is no danger of the needle dropping off the syringe during the transfer process (Fig. 13). Variations in syringe types include those with Teflon® -tipped pistons (plungers), which are somewhat more expensive. Before using a syringe, always check that it is working by sucking up a little of the solvent to be used, ensuring that air is not sucked into the syringe either via the Luer lock or down a gap between the syringe and piston. If all is correct, disassemble the syringe and needle, dry in an oven at 120 °C (not if Teflon® tipped) and allow cooling in a desiccator. Once you have transferred the airsensitive reagent, you must clean out the syringe and needle immediately, by the appropriate method, as air will get into the needle and syringe and decompose the reagent causing the syringe to jam or the needle to block.
*Note: When removing air-sensitive reagents from nitrogenfilled bottles, you must replace the volume of liquid removed with inert gas (nitrogen) from a gas cylinder or balloon, via a needle, otherwise air (water, oxygen and carbon dioxide) will be pulled into the bottle as a result of the vacuum you have created.
Syringes.
Glass, gas-tight syringes with a Luer lock fitting are the most versatile type of syringe, and they come in a range of sizes. The Luer lock enables the stainless-steel needle to be locked in place on the end of the syringe so that there is no danger of the needle dropping off the syringe during the transfer process (Fig. 13). Variations in syringe types include those with Teflon® -tipped pistons (plungers), which are somewhat more expensive. Before using a syringe, always check that it is working by sucking up a little of the solvent to be used, ensuring that air is not sucked into the syringe either via the Luer lock or down a gap between the syringe and piston. If all is correct, disassemble the syringe and needle, dry in an oven at 120 °C (not if Teflon® tipped) and allow cooling in a desiccator. Once you have transferred the airsensitive reagent, you must clean out the syringe and needle immediately, by the appropriate method, as air will get into the needle and syringe and decompose the reagent causing the syringe to jam or the needle to block.
Step-By-Step Procedures.
The techniques shown in this section use balloons of nitrogen gas to create inert atmospheric conditions in a round bottomed flask, and syringes to transfer liquids from dry reagent bottles. These techniques can be easily adapted to use with a gas manifold if available.
Prepare a balloon of inert gas
1. Prepare a needle attachment for a balloon: Cut the end off a plastic 1ml syringe and fit the barrel into a piece of thick rubber tubing. Attach a helium-quality balloon to the rubber tubing, and seal all joints with Parafilm. Alternatively, attach a balloon directly to a 2-3 ml plastic syringe.
2. Fill the balloon by connecting to a hose on the regulator of a tank of inert gas (nitrogen or argon, Fig. 7, a). Open the gas regulator to fill the balloon to between 7" – 8" in diameter (Fig. 7, b). [For use with very sensitive reagents, the gas should first be passed through a column of drying agent.]
3. While holding the balloon close to your body, twist the balloon to prevent gas from escaping. Then attach a green needle (#21 gauge, 0.8 mm×25 mm, safety note: very sharp!) securely to the end of the syringe (Fig. 7, c).
4. To prevent gas from escaping when the balloon is untwisted, insert the needle into a rubber stopper (Fig. 7, d). The balloon can now be set aside while other parts of the setup are prepared.
1. Prepare a needle attachment for a balloon: Cut the end off a plastic 1ml syringe and fit the barrel into a piece of thick rubber tubing. Attach a helium-quality balloon to the rubber tubing, and seal all joints with Parafilm. Alternatively, attach a balloon directly to a 2-3 ml plastic syringe.
2. Fill the balloon by connecting to a hose on the regulator of a tank of inert gas (nitrogen or argon, Fig. 7, a). Open the gas regulator to fill the balloon to between 7" – 8" in diameter (Fig. 7, b). [For use with very sensitive reagents, the gas should first be passed through a column of drying agent.]
3. While holding the balloon close to your body, twist the balloon to prevent gas from escaping. Then attach a green needle (#21 gauge, 0.8 mm×25 mm, safety note: very sharp!) securely to the end of the syringe (Fig. 7, c).
4. To prevent gas from escaping when the balloon is untwisted, insert the needle into a rubber stopper (Fig. 7, d). The balloon can now be set aside while other parts of the setup are prepared.
Prepare the reagent flask
5. Remove the surface water from a reagent flask (with stir bar, if applicable), by either flame drying the flask or placing it in a hot oven for several hours. Safety note: flask will be very hot! Use thick gloves to handle the hot glass.
6. Immediately insert a rubber septum (Fig. 8, a) into the ground glass joint. Fold one side of the septum over the lip of the flask and hold it in place while folding the opposite sides over as well (Fig. 8, b-d). This can be difficult to do with thick gloves. An alternative is to hold the flask against your body with the thick gloves, and fold the septum flaps over while using your bare hands (or thinner gloves, Fig. 9, a+b).
7. Immediately secure the reaction flask to a ring stand or latticework using an extension clamp and insert the needle of the inert gas balloon into the inner circle on the septum (Fig. 9 c, see Fig. 8 d for the circle on the septum).
8. Insert a single needle into the circle on the septum (called an "exit needle") to "flush" the air from the reaction flask (Fig. 9 d). The goal is to use the pressure from the balloon to force inert gas into the reaction flask and displace the air in the flask out the exit needle.
9. Allow the system to flush for at least 5 minutes if using nitrogen gas and perhaps 1-2 minutes if using argon gas (argon is denser than air so will displace the air more easily than nitrogen). Then remove the exit needle and allow the flask to fully cool under the balloon of inert gas.
5. Remove the surface water from a reagent flask (with stir bar, if applicable), by either flame drying the flask or placing it in a hot oven for several hours. Safety note: flask will be very hot! Use thick gloves to handle the hot glass.
6. Immediately insert a rubber septum (Fig. 8, a) into the ground glass joint. Fold one side of the septum over the lip of the flask and hold it in place while folding the opposite sides over as well (Fig. 8, b-d). This can be difficult to do with thick gloves. An alternative is to hold the flask against your body with the thick gloves, and fold the septum flaps over while using your bare hands (or thinner gloves, Fig. 9, a+b).
7. Immediately secure the reaction flask to a ring stand or latticework using an extension clamp and insert the needle of the inert gas balloon into the inner circle on the septum (Fig. 9 c, see Fig. 8 d for the circle on the septum).
8. Insert a single needle into the circle on the septum (called an "exit needle") to "flush" the air from the reaction flask (Fig. 9 d). The goal is to use the pressure from the balloon to force inert gas into the reaction flask and displace the air in the flask out the exit needle.
9. Allow the system to flush for at least 5 minutes if using nitrogen gas and perhaps 1-2 minutes if using argon gas (argon is denser than air so will displace the air more easily than nitrogen). Then remove the exit needle and allow the flask to fully cool under the balloon of inert gas.
10. If a mass is required of the empty flask, remove the inert gas balloon (insert the needle into a rubber stopper) and obtain the mass of the cool, empty flask with septum.
Prepare the syringe for reagent transfer
11. Remove a long, flexible needle from a hot oven and immediately screw it into the barrel of a plastic syringe, freshly opened from its packaging (Fig.10 a). The syringe needs to be able to hold a volume larger than the volume of reagent intended to deliver to have enough flexibility to properly manipulate the reagent. For example, a 10 ml syringe is too small to deliver 10 ml of reagent, but could be used to deliver 7 ml of reagent. Hold the syringe such that the volume markings are visible, and connect the bent needle pointed upwards, so that when screwed on (which normally requires roughly a half turn) the bent needle points downwards with the numbers visible. With this approach, the volume markings can be seen while withdrawing liquid, instead of being inconveniently on the back face of the syringe (as in Fig.10 d). Glass syringes are often used with air-sensitive reagents dissolved in nonpolar solvents (e.g. hexanes), and require some further considerations that are not described in this section. Consult with your instructor for further instructions if you are to use a glass syringe.
12. Wrap the joint between the needle and syringe with Teflon tape or Parafilm (Fig.10 b).
13. Flush the needle with inert gas: Insert the needle into the septum of an empty, dry flask attached to a balloon of inert gas (Fig.10 c), withdraw a full volume of inert gas (Fig.10 d), then expunge it into the air.
14. Immediately insert the flushed syringe into the reagent flask septum if nearby, or into a rubber stopper until the syringe is to be used.
11. Remove a long, flexible needle from a hot oven and immediately screw it into the barrel of a plastic syringe, freshly opened from its packaging (Fig.10 a). The syringe needs to be able to hold a volume larger than the volume of reagent intended to deliver to have enough flexibility to properly manipulate the reagent. For example, a 10 ml syringe is too small to deliver 10 ml of reagent, but could be used to deliver 7 ml of reagent. Hold the syringe such that the volume markings are visible, and connect the bent needle pointed upwards, so that when screwed on (which normally requires roughly a half turn) the bent needle points downwards with the numbers visible. With this approach, the volume markings can be seen while withdrawing liquid, instead of being inconveniently on the back face of the syringe (as in Fig.10 d). Glass syringes are often used with air-sensitive reagents dissolved in nonpolar solvents (e.g. hexanes), and require some further considerations that are not described in this section. Consult with your instructor for further instructions if you are to use a glass syringe.
12. Wrap the joint between the needle and syringe with Teflon tape or Parafilm (Fig.10 b).
13. Flush the needle with inert gas: Insert the needle into the septum of an empty, dry flask attached to a balloon of inert gas (Fig.10 c), withdraw a full volume of inert gas (Fig.10 d), then expunge it into the air.
14. Immediately insert the flushed syringe into the reagent flask septum if nearby, or into a rubber stopper until the syringe is to be used.
Withdraw the reagent.
15. A balloon of inert gas must be inserted into the reagent bottle in order to equalize pressures during withdrawal of liquid. A platform (e.g. ring clamp/wire mesh) should also be used beneath the reagent bottle if positioned above the bench, to provide support in case the bottle slips from the grasp of the clamp.16. Insert the needle of the flushed syringe into the septum of the air-sensitive reagent, and into the liquid (Fig.11 a).
17. Slowly withdraw some liquid into the syringe. If the plunger is pulled back too quickly, the low-pressure inside the syringe may cause air to seep through the joint between the needle and syringe (through or around the Teflon tape or Parafilm).
18. Inevitably, a bubble will form in the syringe. Keeping the syringe upside down and vertical (Fig.11 b), push on the plunger to force the gas pocket back into the bottle.
19. Slowly withdraw liquid to 1-2 ml greater than the desired volume (Fig.11 c), then keeping the syringe vertical, expunge liquid back to the desired volume (Fig.11 d shows 2.0 ml of liquid). Withdrawing greater than the desired volume at first allows you to be confident that no gas bubbles are in the needle, and that you have measured an accurate volume.
20. The needle should be full of the air-sensitive reagent at this point, and if it were removed from the bottle, the reagent would come into contact with the atmosphere at the needle tip. This can have disastrous consequences if the reagent is quite reactive (smoking or potentially fire). Safety note: It is therefore essential that a "buffer" of inert gas (Fig.13) is placed between the air-sensitive reagent and the atmosphere before removing the needle.
17. Slowly withdraw some liquid into the syringe. If the plunger is pulled back too quickly, the low-pressure inside the syringe may cause air to seep through the joint between the needle and syringe (through or around the Teflon tape or Parafilm).
18. Inevitably, a bubble will form in the syringe. Keeping the syringe upside down and vertical (Fig.11 b), push on the plunger to force the gas pocket back into the bottle.
19. Slowly withdraw liquid to 1-2 ml greater than the desired volume (Fig.11 c), then keeping the syringe vertical, expunge liquid back to the desired volume (Fig.11 d shows 2.0 ml of liquid). Withdrawing greater than the desired volume at first allows you to be confident that no gas bubbles are in the needle, and that you have measured an accurate volume.
20. The needle should be full of the air-sensitive reagent at this point, and if it were removed from the bottle, the reagent would come into contact with the atmosphere at the needle tip. This can have disastrous consequences if the reagent is quite reactive (smoking or potentially fire). Safety note: It is therefore essential that a "buffer" of inert gas (Fig.13) is placed between the air-sensitive reagent and the atmosphere before removing the needle.
21. To create the "inert gas buffer":
a. Place the needle into the headspace of the reagent bottle (Fig.12 a+b).
b. Keeping the syringe upside down and vertical, gently pull back on the plunger until a bubble is seen in the barrel (approximately 20% of the syringe capacity, Fig.12 c). Immediately insert the syringe into the reaction flask septum if nearby, or into a rubber stopper if the flask is a distance away (Fig.12 d).
Deliver the reagent.
22. With an inert gas balloon inserted in the reaction flask, place the syringe with reagent into the reaction flask septum. Keeping the syringe vertical, push on the plunger to first deliver the inert gas buffer (Fig.14 a), then slowly deliver reagent to the flask.
23. Stop delivering reagent when the rubber plunger of the syringe meets the end of the barrel (Fig.14 b). Do not invert the syringe and push out the residual liquid: this would result in delivering a larger volume of reagent than measured by the syringe.
24. The needle will still be full of the air-sensitive reagent, so with the needle tip still in the headspace of the reaction flask, withdraw an inert gas buffer into the syringe. Insert the needle tip into a rubber stopper if the cleaning station is not nearby.
22. With an inert gas balloon inserted in the reaction flask, place the syringe with reagent into the reaction flask septum. Keeping the syringe vertical, push on the plunger to first deliver the inert gas buffer (Fig.14 a), then slowly deliver reagent to the flask.
23. Stop delivering reagent when the rubber plunger of the syringe meets the end of the barrel (Fig.14 b). Do not invert the syringe and push out the residual liquid: this would result in delivering a larger volume of reagent than measured by the syringe.
24. The needle will still be full of the air-sensitive reagent, so with the needle tip still in the headspace of the reaction flask, withdraw an inert gas buffer into the syringe. Insert the needle tip into a rubber stopper if the cleaning station is not nearby.
Clean the needle and syringe.
25. The syringe and needle should be cleaned as soon as possible, as over time deposits may form in the needle, creating a plug. To clean the syringe and needle:
25. The syringe and needle should be cleaned as soon as possible, as over time deposits may form in the needle, creating a plug. To clean the syringe and needle:
a. Withdraw into the syringe a few ml of clean solvent similar to the solvent used in the air-sensitive solution (Fig.14 c). For example, the pictures in this section show transfer of a BH3 reagent dissolved in THF. An ideal rinse solvent would then be THF. As THF was not available, diethyl ether was a good substituted as the two solvents are structurally similar (they are both ethers).
b. Expunge the solvent into a waste beaker. Repeat with another solvent rinse, being sure to rinse the entire area in the syringe where the reagent touched.
c. Rinse the syringe once with water to dissolve and remove any inorganic salts.
d. Further, rinse the syringe and needle twice with a few ml of acetone.
e. Remove the needle from the syringe and retain for future use. The plastic syringe should not be reused, but instead thrown away: solvent present in many air-sensitive solutions degrade the rubber plunger on the syringe, causing them to swell and be ineffective after one use.
Syringe needles and cannulae.
Stainless steel Luer lock syringe needles come in various lengths and diameters. The length of needle you will need depends on the size of the vessel from which you wish to withdraw the liquid; the diameter required depends on the size of the syringe - you should not use a large-diameter needle with a small-volume syringe - and the viscosity of the solution or liquid. Needle diameters are expressed in 'gauge': the higher the gauge, the narrower the needle diameter. For most inert atmosphere work, you should use a needle with a 'non-coring' or 'deflecting' tip (Fig. 15), which ensures that a piece of the septum is not trapped in the needle when you push it through.
Stainless steel Luer lock syringe needles come in various lengths and diameters. The length of needle you will need depends on the size of the vessel from which you wish to withdraw the liquid; the diameter required depends on the size of the syringe - you should not use a large-diameter needle with a small-volume syringe - and the viscosity of the solution or liquid. Needle diameters are expressed in 'gauge': the higher the gauge, the narrower the needle diameter. For most inert atmosphere work, you should use a needle with a 'non-coring' or 'deflecting' tip (Fig. 15), which ensures that a piece of the septum is not trapped in the needle when you push it through.
Cannulae are long, flexible, double-ended needles made from stainless steel or inert plastics, which are used to transfer large volumes of reagents or solvents from one vessel to another under inert gas pressure (Fig. 16).
Summary
Many experimental set-ups are possible with three-necked flasks and glassware with an additional tap or connector to provide an inlet for the inert gas and access to the vacuum for the evacuation and refill cycle. Solids, whether air-sensitive or air-stable, should be added at the beginning of the reaction if possible. Adding solids mid-way through a reaction is trickier but can be done with solid addition funnel or tube. Liquids are added with a syringe if the quantity is under 50 ml or via a cannula if more than 50 ml is required.
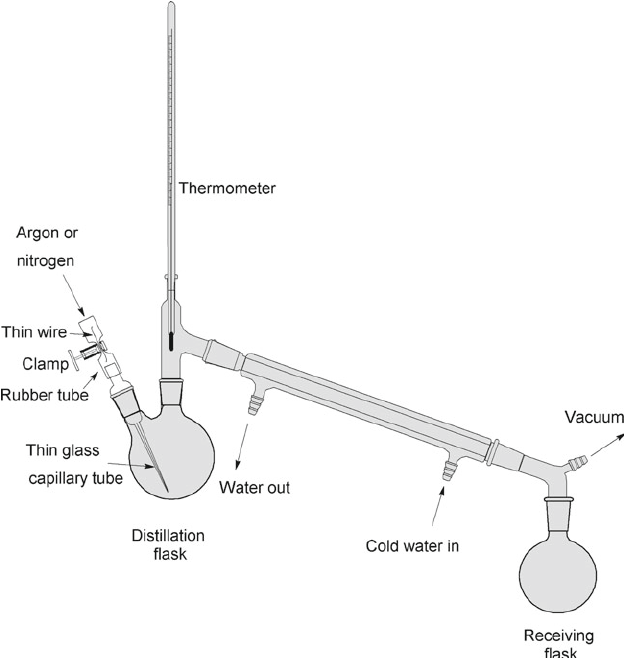
Dynamic air-free distillation
Dynamic air-free vacuum distillations are routinely employed to purify high-boiling liquids (>150 °C), air sensitive substances and some low-melting solids. This method is well suited for commercially available reagents or compounds prepared on a large scale in the laboratory in which the (often known) impurities are non-volatile and so remain behind after distillation.
Fig.18
Step-By-Step Procedures
Step 1: The impure material is transferred to a suitable Schlenk flask equipped with a magnetic stir bar. For commercially available reagents to be purified in bulk, this can be added to a Schlenk flask (that has already been cycled onto the inert gas line) under a flow of inert gas. For “in-house” prepared compounds, the crude material will typically remain in the Schlenk flask after removing the solvent and volatiles in vacuo.
Step 2: A Schlenk flask, distillation bridge and Schlenk cap are greased, assembled and cycled onto the inert gas line. Note: The style of distillation bridge (single-piece) illustrated is designed specifically to purify high-boiling liquids under high-vacuum. This differs from a typical distillation setup with a distillation head, thermometer adapter, and water-cooled condenser.
Step 3: Once the receiving Schlenk flask and distillation bridge has been cycled onto the line and back-filled with inert gas, it is connected to the Schlenk flask containing the crude material. This may require a brief helping hand to remove clips and stoppers. Ensure that inert gas is flowing into both flasks during this process to minimise exposure to atmospheric air and moisture.
Step 4: The stopcocks on both Schlenk flasks are closed and the distillation flask is lowered into a suitable heating mantle or oil bath. With stirring, the stopcock on the receiving Schlenk flask is slowly and carefully opened to vacuum. This serves to degas the crude material and remove any residual solvent or volatile impurities. Note: The bulk material should be sufficiently high-boiling so that it does not evaporate at ambient temperatures whilst under vacuum. An external liquid nitrogen trap can be used between the receiving flask and inert gas line to condense any volatile compounds.
Step 5: Once a good vacuum (i.e. low pressure) has been established within the distillation setup, and the crude material is fully degassed, the temperature on the heating mantle can slowly be increased. Since the crude material does not evaporate at ambient temperature, an ice bath is generally sufficient to cool the receiving flask and condense the distillate, however a dewar of liquid nitrogen can also be used. During the distillation, it may be necessary to insulate the flask and part of the bridge with aluminium foil, or to briefly heat the glassware with a heat-gun.
Step 6: Once the distillation is complete, the stopcock is closed on the receiving flask. The heating mantle is lowered to allow the distillation flask to cool to ambient temperature, and the cooling bath is removed from the receiving flask to allow it to thaw or warm to ambient temperature.
Step 7: When the distillation apparatus is at room temperature, the system is slowly backfilled with inert gas. If an external trap was used, it is necessary to disconnect this first and cycle the receiving flask back onto the inert gas line.
Step 8: Under a flow of inert gas, the distillation bridge can be removed from the receiving flask and replaced with a clean, greased ground-glass stopper. The purified material can now be transferred to a suitable ampoule for storage via cannula transfer, or used directly for further manipulations.
Hints and tips:
- An approximate distillation temperature can be calculated using the known boiling point of the compound (at ambient pressure) and the pressure within the inert gas line (if a manometer is being used).
- For complex mixtures of species to be separated by vacuum distillation, a more elaborate setup containing a Vigreux column, a thermometer adapter, and a "pig" receiver (spider or cow receivers) is generally required to collect multiple fractions.
Last edited by a moderator:
