Introduction.
Washing laboratory glassware belongs to the mandatory list of skills and abilities of laboratory personnel. High-quality washing implies the achievement of the main result - good cleanliness of chemical glassware for perfect organic synthesis. A number of requirements are put forward for laboratory glassware, since it must always be perfectly clean. It is recommended to wash laboratory glassware immediately after use. The results of the work performed will depend on the cleanliness of the dishes. It's generally easier to clean glassware if you do it right away. When a detergent is used, it's usually one designed for lab glassware, such as Liquinox or Alconox. These detergents are preferable to any dishwashing detergent that might be used on dishes at home. Typically, detergent and tap water are neither required nor desirable. You can rinse the glassware with the proper solvent, then finish up with a couple of rinses with distilled water, followed by final rinses with deionized water.
The best way to clean up laboratory glassware is using some aggressive detergent which will oxydize pollutions or/and remove them easy. There is a list of detergents, synthesis instructions and use manuals.
The best way to clean up laboratory glassware is using some aggressive detergent which will oxydize pollutions or/and remove them easy. There is a list of detergents, synthesis instructions and use manuals.
Sulfochromic mixture.
It is a mixture of concentrated sulfuric acid and potassium dichromate; when sulfuric acid acts on dichromate, chromic anhydride CrO3 is formed. Sulfochromic mixture is one of the strongest oxidizing agents. It is widely used in laboratory technology for washing chemical glassware, and also applied in the bleaching process of reversible photography.
A solution of chromic acid in sulfuric acid (also known as a sulfochromic mixture or chromosulfuric acid) is a powerful oxidizing agent, it can be used to clean laboratory glassware, particularly of otherwise insoluble organic residues. Furthermore, the acid leaves trace amounts of paramagnetic chromic ions — Cr(III) — that can interfere with certain applications, such as NMR spectroscopy. This is especially the case for NMR tubes.
You need:
You need:
- Potassium/sodium dichromate (K₂Cr₂O₇/Na₂Cr₂O₇) - 15 g
- Concentrated sulfuric (or nitric) acid (H2SO4/HNO3) - 500 ml
To prepare the chromium mixture, carefully while stirring, potassium dichromate is slowly added to the sulfuric acid. The mixture gets very hot, and the solution turns dark brown. Potassium dichromate is poorly soluble, long stirring with a glass rod is required. The procedure should be carried out in a ceramic glass in a water (cold) bath to remove heat. If sediment remains at the bottom, then don't worry, it should be so.
Application.
Glassware is rinsed with running water, then poured with a chromium mixture and kept for several minutes, if necessary - a couple of days, and then thoroughly washed in running water. On well-degreased glass, water spreads in a thin layer, without collecting into drops. The chromium mixture can be reused until the color changes to greenish. As a result of the ongoing oxidation reactions of organic substances, chromic anhydride is reduced to chromium (III) sulfate, as a result of which the used chromium mixture gradually changes its color to green.
Safety.
Concentrated sulfuric acid is a highly corrosive substance! Pour only acid into water! Chromium mixture is also corrosive; in addition, hexavalent chromium compounds are toxic and carcinogenic. When you are working with a chromium mixture, you should observe safety precautions and use personal protective equipment. Store the mixture in a fume cupboard or in a tightly closed container (not a rubber stopper!). When washing pipettes and various tubes, the mixture should be collected only with a rubber bulb, in no case with the mouth, to avoid severe burns of the mouth and damage to teeth.
Application.
Glassware is rinsed with running water, then poured with a chromium mixture and kept for several minutes, if necessary - a couple of days, and then thoroughly washed in running water. On well-degreased glass, water spreads in a thin layer, without collecting into drops. The chromium mixture can be reused until the color changes to greenish. As a result of the ongoing oxidation reactions of organic substances, chromic anhydride is reduced to chromium (III) sulfate, as a result of which the used chromium mixture gradually changes its color to green.
Safety.
Concentrated sulfuric acid is a highly corrosive substance! Pour only acid into water! Chromium mixture is also corrosive; in addition, hexavalent chromium compounds are toxic and carcinogenic. When you are working with a chromium mixture, you should observe safety precautions and use personal protective equipment. Store the mixture in a fume cupboard or in a tightly closed container (not a rubber stopper!). When washing pipettes and various tubes, the mixture should be collected only with a rubber bulb, in no case with the mouth, to avoid severe burns of the mouth and damage to teeth.
Potassium permanganate solution.
You need:
- Potassium permanganate (KMnO4)
- Oxalic acid/ sodium hydrogen sulfate/ FeSO4/ Mohr's salt
Application.
A good laboratory glassware detergent is a 4% potassium permanganate solution. Potassium permanganate solution is a strong oxidizing agent, especially when heated and acidified with sulfuric acid; it is poured into dishes, which must first be washed with hot water and cleaned with a special brush. Then a small amount of concentrated sulfuric acid is carefully added, which causes heating, quite enough so that all impurities on the walls are quickly oxidized. Sulfuric acid should be taken in such an amount that after adding its solution temperature was about 50-60 °C. Usually, it is enough to add 3-5 ml of concentrated sulfuric acid to 100 ml of potassium permanganate solution. It is necessary to take sulfuric acid and in no case hydrochloric acid, since the latter is oxidized by potassium permanganate with the formation of chlorine. Brown plaque may appear after washing laboratory glassware with a solution of potassium permanganate. It can be removed by rinsing the dishes with a 5% solution of sodium hydrogen sulfate (NaHSO4), solutions of iron (II) sulfate (FeSO4), Mohr's salt or organic acids, preferably oxalic acid. After that, the dishes are washed with water.
The spent acidified potassium permanganate solution is usually discarded and not reused. If a non-acidified solution was used, it can be used several times.
I would recommend you to make big (3-5 l) bath for laboratory glassware, use empty desiccator or another glass or ceramic dish. You can lay dirty glassware into this bath for 2-3 h for oxidizing pollutions on glass walls (previously clean them up by brush and tap water). After, clean this glassware from potassium permanganate solution by tap water and lay for 0.5-1 h into oxalic acid bath to remove manganese oxides (brown plaque). After, repeat procedure with tap water and with distilled water.
Safety.
The same cleaning practices and precautions for handling acidified potassium permanganate solution as described above for the sulfochromic mixture should be followed.
A good laboratory glassware detergent is a 4% potassium permanganate solution. Potassium permanganate solution is a strong oxidizing agent, especially when heated and acidified with sulfuric acid; it is poured into dishes, which must first be washed with hot water and cleaned with a special brush. Then a small amount of concentrated sulfuric acid is carefully added, which causes heating, quite enough so that all impurities on the walls are quickly oxidized. Sulfuric acid should be taken in such an amount that after adding its solution temperature was about 50-60 °C. Usually, it is enough to add 3-5 ml of concentrated sulfuric acid to 100 ml of potassium permanganate solution. It is necessary to take sulfuric acid and in no case hydrochloric acid, since the latter is oxidized by potassium permanganate with the formation of chlorine. Brown plaque may appear after washing laboratory glassware with a solution of potassium permanganate. It can be removed by rinsing the dishes with a 5% solution of sodium hydrogen sulfate (NaHSO4), solutions of iron (II) sulfate (FeSO4), Mohr's salt or organic acids, preferably oxalic acid. After that, the dishes are washed with water.
The spent acidified potassium permanganate solution is usually discarded and not reused. If a non-acidified solution was used, it can be used several times.
I would recommend you to make big (3-5 l) bath for laboratory glassware, use empty desiccator or another glass or ceramic dish. You can lay dirty glassware into this bath for 2-3 h for oxidizing pollutions on glass walls (previously clean them up by brush and tap water). After, clean this glassware from potassium permanganate solution by tap water and lay for 0.5-1 h into oxalic acid bath to remove manganese oxides (brown plaque). After, repeat procedure with tap water and with distilled water.
Safety.
The same cleaning practices and precautions for handling acidified potassium permanganate solution as described above for the sulfochromic mixture should be followed.
Alkali alcohol solution.
The alcohol-hydroxide cleanser is used to clean glass. It is an effective cleaner.
You need:
You need:
- 60 g sodium hydroxide (NaOH)/potassium hydroxide (KOH);
- 500 ml ethanol;
- 60 ml DI water;
- Polypropylene or glass container (600 ml or greater).
Prepare the sodium hydroxide solution by mixing the crystals into the water. Then add the ethanol. The mixture gets very hot, be careful. You have to stir the solution with a glass rod until dissolution is complete. Be sure to label the container with the title “Ethanol/NaOH 5:1 cleaning solution”.
Application.
Put the laboratory glassware into the bath to soak for 30 minutes. For spotless surfaces, soak for several hours. Rinse in DI water then blow dry. If cleaning solution is clean, and you intend to use it again, store it in an appropriately labeled container. If not, pour the alkaline solution into the appropriately labeled waste container.
Safety
Wear protective gear: eye protection, chemical coat and nitrile gloves.
Application.
Put the laboratory glassware into the bath to soak for 30 minutes. For spotless surfaces, soak for several hours. Rinse in DI water then blow dry. If cleaning solution is clean, and you intend to use it again, store it in an appropriately labeled container. If not, pour the alkaline solution into the appropriately labeled waste container.
Safety
Wear protective gear: eye protection, chemical coat and nitrile gloves.
To clean glassware, use the following procedures:
1. Use 2-3 mL solvent to rinse residual organic compounds from the glassware into a waste beaker. The compounds should be highly soluble in the solvent. The default solvent is often acetone as it is inexpensive, relatively nontoxic, and dissolves most organic compounds. Some institutions reuse their acetone ("wash acetone") as the solvation ability is not spent after a few uses.
2. As it will soon become second-nature for most students to use acetone as part of their cleaning ritual, it is worth reminding that the purpose of acetone rinse is to dissolve organic residue in a flask. Not everything dissolves in acetone, for example ionic salts are insoluble in acetone and are more successfully rinsed out with water. After a preliminary rinse, glassware should then be washed with soap and water at the bench.
3. Residual acetone will likely evaporate from the flask, but it is acceptable for small quantities of residual acetone to be washed down the drain. Acetone is a normal biological byproduct of some metabolic processes.
4. If using undiluted detergent from the store, it is best to use small amounts during washing, as they tend to form thick foams that need lots of rinsing. Some institutions instead use dilute soap solutions at their cleaning stations for this reason. For cleaning of glassware, the biodegradable detergent "Alconox" is the industry standard.
This step can be replaced with another one from the list of detergents above. Soap cannot wash any organic substances and one day you will be faced with remains of dirt. The best way is Sulfochromic mixture, which can oxidize almost all organic substances for appropriate exposition time. If you can't find so a lot of sulfuric acid/nitric acid, I would recommend you take Alkali alcohol solution. It is cheap and easy to produce cleaning solution. Potassium permanganate solution is more effectively cope with organic pollutions, but it is quite difficult, and you have to clean up a glassware from manganese oxides by helps of second solution of Oxalic acid, which have to be removed by tap or distillated water.
2. As it will soon become second-nature for most students to use acetone as part of their cleaning ritual, it is worth reminding that the purpose of acetone rinse is to dissolve organic residue in a flask. Not everything dissolves in acetone, for example ionic salts are insoluble in acetone and are more successfully rinsed out with water. After a preliminary rinse, glassware should then be washed with soap and water at the bench.
3. Residual acetone will likely evaporate from the flask, but it is acceptable for small quantities of residual acetone to be washed down the drain. Acetone is a normal biological byproduct of some metabolic processes.
4. If using undiluted detergent from the store, it is best to use small amounts during washing, as they tend to form thick foams that need lots of rinsing. Some institutions instead use dilute soap solutions at their cleaning stations for this reason. For cleaning of glassware, the biodegradable detergent "Alconox" is the industry standard.
This step can be replaced with another one from the list of detergents above. Soap cannot wash any organic substances and one day you will be faced with remains of dirt. The best way is Sulfochromic mixture, which can oxidize almost all organic substances for appropriate exposition time. If you can't find so a lot of sulfuric acid/nitric acid, I would recommend you take Alkali alcohol solution. It is cheap and easy to produce cleaning solution. Potassium permanganate solution is more effectively cope with organic pollutions, but it is quite difficult, and you have to clean up a glassware from manganese oxides by helps of second solution of Oxalic acid, which have to be removed by tap or distillated water.
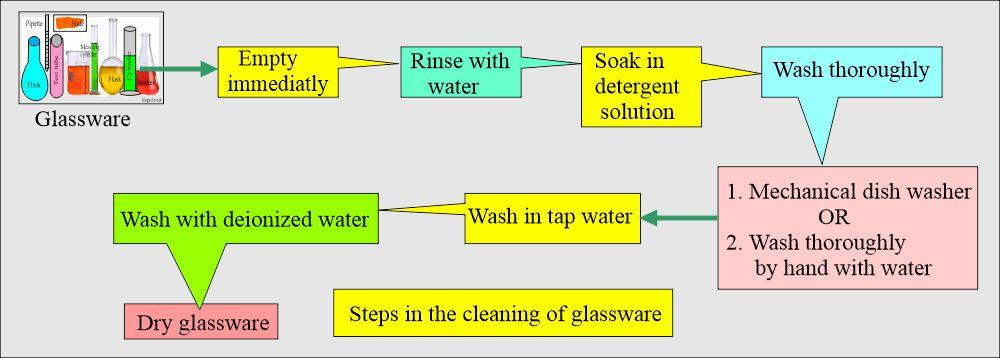
Steps in the washing of the laboratory glassware:
Automatic dishwasher.
In an underground laboratory, automatic dishwasher can be used as well. But there are a number of problems which can appear with organic or aggressive substances. Plastic membranes, filters, rubbers will crack after some hundred hours of works. Metal surface will rust in acidly environment. To avoid these issues and prolong dishwasher life, you have to rinse glassware by tap water before load it. Your dishwasher will break with a high probability, but if you accept this cost for clean glassware, you can use it. In case of large laboratory load, it helps to safe a lot of time.
Drying Glassware.
Quick Drying.If dry glassware is not needed right away, it should be rinsed with distilled water and allowed to dry overnight (in a locker). If dry glassware is promptly needed, glassware can be rinsed with acetone and the residual acetone allowed to evaporate. Rinsing with acetone works well because water is miscible with acetone, so much of the water is removed in the rinse waste. Evaporation of small amounts of residual acetone can be expedited by placing the rinsed glassware in a warm oven for a short amount of time, or by using suction from a tube connected to the water aspirator. Residual acetone should not be evaporated inside a hot oven (>100 °C) as acetone may polymerize and/or ignite under these conditions. It should also not be evaporated using the house compressed air lines, as this is likely to contaminate the glassware with dirt, oil, and moisture from the air compressor.
Oven and Flame Drying.
Glassware that appears "dry" actually contains a thin film of water condensation on its surface. When using reagents that react with water (sometimes violently!), this water layer needs to be removed. To evaporate the water film, glassware can be placed in a 110 °C oven overnight, or at the least for several hours. The water film can also be manually evaporated using a burner or heat gun, a process called "flame drying". Both methods result in extremely hot glassware that must be handled carefully with tongs or thick gloves.
Oven and Flame Drying.
Glassware that appears "dry" actually contains a thin film of water condensation on its surface. When using reagents that react with water (sometimes violently!), this water layer needs to be removed. To evaporate the water film, glassware can be placed in a 110 °C oven overnight, or at the least for several hours. The water film can also be manually evaporated using a burner or heat gun, a process called "flame drying". Both methods result in extremely hot glassware that must be handled carefully with tongs or thick gloves.
To flame dry glassware, first remove any vinyl sleeves on an extension clamp (Fig.1 a), as these can melt or catch on fire. Clamp the flask to be dried, including a stir bar if using (Fig.1 b). Apply the burner or heat gun to the glass, and initially fog will be seen as water vaporizing from one part of the glassware condenses elsewhere (Fig.1 c). Continue waving the heat source all over the glassware for several minutes until the fog is completely removed, and the glassware is scorching hot (Fig.1 d). If the glass is only moderately hot, water will condense from the air before you can fully exclude it.
Safety Note: glassware will be extremely hot after flame drying.
Regardless of the manner in which glassware is heated (oven or flame drying), allow the glassware to cool in a water-free environment (in a desiccator, under a stream of inert gas, or with a drying tube, Fig. 2) before obtaining a mass or adding reagents.
Safety Note: glassware will be extremely hot after flame drying.
Regardless of the manner in which glassware is heated (oven or flame drying), allow the glassware to cool in a water-free environment (in a desiccator, under a stream of inert gas, or with a drying tube, Fig. 2) before obtaining a mass or adding reagents.
Drying Tubes.
A drying tube is used when moderately but not meticulously dry conditions are desired in an apparatus. If meticulously dry conditions are necessary, glassware should be oven or flame dried, then the air displaced with a dry, inert gas.
Drying tubes are pieces of glassware that can be filled with a drying agent (often anhydrous CaCl2 or CaSO4 in the pellet form) and connected to an apparatus either through a thermometer adapter (Fig. 3 b and c) or rubber tubing (Fig.3 d). Air passing through the tube is removed of water when it comes in contact with the drying agent. Since it is important that air can flow through the drying tube, especially so the apparatus is not a closed system, the drying agent should be fresh as used drying agents can sometimes harden into a plug that restricts airflow. Drying tubes can also be filled with basic solids such as Na2CO3 to neutralize acidic gases.
A drying tube is used when moderately but not meticulously dry conditions are desired in an apparatus. If meticulously dry conditions are necessary, glassware should be oven or flame dried, then the air displaced with a dry, inert gas.
Drying tubes are pieces of glassware that can be filled with a drying agent (often anhydrous CaCl2 or CaSO4 in the pellet form) and connected to an apparatus either through a thermometer adapter (Fig. 3 b and c) or rubber tubing (Fig.3 d). Air passing through the tube is removed of water when it comes in contact with the drying agent. Since it is important that air can flow through the drying tube, especially so the apparatus is not a closed system, the drying agent should be fresh as used drying agents can sometimes harden into a plug that restricts airflow. Drying tubes can also be filled with basic solids such as Na2CO3 to neutralize acidic gases.
Conclusion and Important facts:
- Should clean the glassware as soon as possible.
- Regarding delay, put the glassware in water.
- As to late cleaning, the residue may not be possible to remove.
- New glassware that is slightly alkaline needs to be soaked in acid water (1% HCl or HNO3) for several hours before washing.
This topic contains the list of most common glassware cleaning techniques. I do not suggest extremely dangerous washing solutions, such as Piranha and so on here because they can cause burns, fire, explosion in low skill chemist arms. Use personal protection in every use of these aggressive detergents and keep your glassware clean, good luck!
Last edited by a moderator:
