- Joined
- Dec 23, 2022
- Messages
- 42
- Reaction score
- 28
- Points
- 18
Hey guys, a while back, I delved into the German New Psychoactive Substances Act and noticed that many substance groups are listed there, but no tropane alkaloids. This led me to the idea that any changes to the cocaine molecule should make these substances completely legal (at least in Germany). For example: 4-fluorococaine...
However, I encountered a problem, namely that the tropane backbone is not so easy to replicate. So, I began to search for suitable precursor substances and came across methyl ecgonidine. But since I couldn't find a source for this substance anywhere, I wondered if the carbon bridge in the tropane structure is really necessary, or if it can simply be omitted, resulting in a piperidine backbone.
I then attempted this with methyl ecgonidine and came across a substance called arecoline, which could be easily extracted from betel nuts or synthesized. After some research, I found a paper that addressed this exact topic: "Synthesis of Cocaine analogs from Arecoline."
In this paper, an RTI-31 analog based on piperidine from arecoline was synthesized, which seems to actually work, as I also found this substance on Wikipedia as a substitute for cocaine addicts. The effectiveness of these "phenylpiperidines" seems to be confirmed to some extent.
So, it seems possible to create various cocaine or troparil analogs based on piperidine, which has led me to ideas like the following (but this could likely apply to various cocaine derivatives as well):
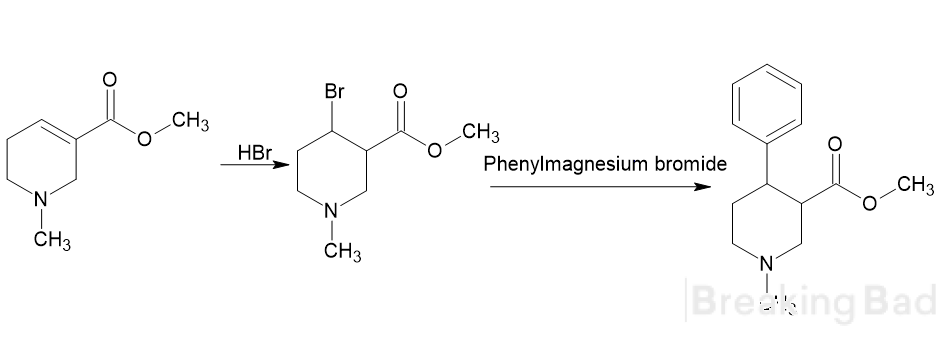
or this one
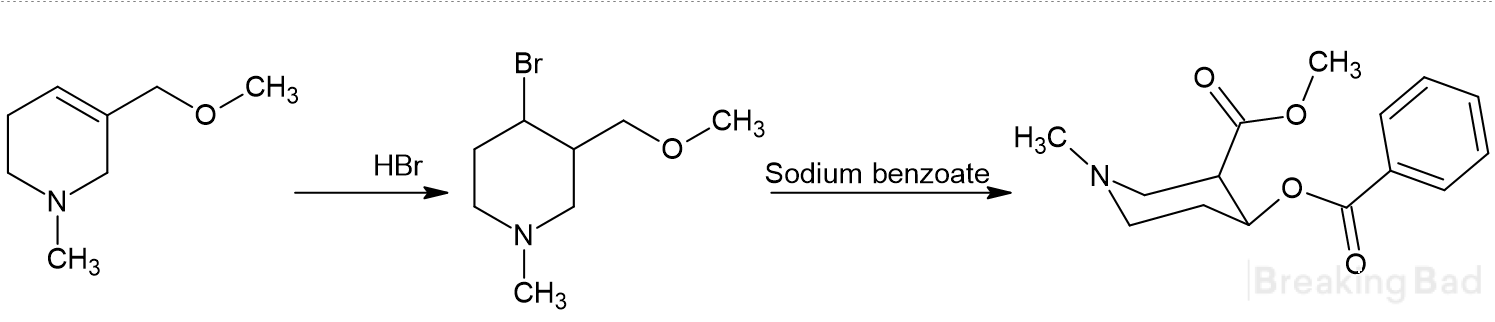
What do you think of the idea? I believe that this group of substances could potentially yield a lot of interesting and effective compounds.
However, I encountered a problem, namely that the tropane backbone is not so easy to replicate. So, I began to search for suitable precursor substances and came across methyl ecgonidine. But since I couldn't find a source for this substance anywhere, I wondered if the carbon bridge in the tropane structure is really necessary, or if it can simply be omitted, resulting in a piperidine backbone.
I then attempted this with methyl ecgonidine and came across a substance called arecoline, which could be easily extracted from betel nuts or synthesized. After some research, I found a paper that addressed this exact topic: "Synthesis of Cocaine analogs from Arecoline."
In this paper, an RTI-31 analog based on piperidine from arecoline was synthesized, which seems to actually work, as I also found this substance on Wikipedia as a substitute for cocaine addicts. The effectiveness of these "phenylpiperidines" seems to be confirmed to some extent.
So, it seems possible to create various cocaine or troparil analogs based on piperidine, which has led me to ideas like the following (but this could likely apply to various cocaine derivatives as well):
or this one
What do you think of the idea? I believe that this group of substances could potentially yield a lot of interesting and effective compounds.
