- Language
- 🇫🇷
- Joined
- Apr 10, 2022
- Messages
- 303
- Reaction score
- 174
- Points
- 43
Hello;
I am currently preparing my notes for future synthesis and I have a question about the choroform synthesis
I have this product:

it says 13% so I'm doing my calculations... (if i was good)
right?
but then I see in the datasheet these details:
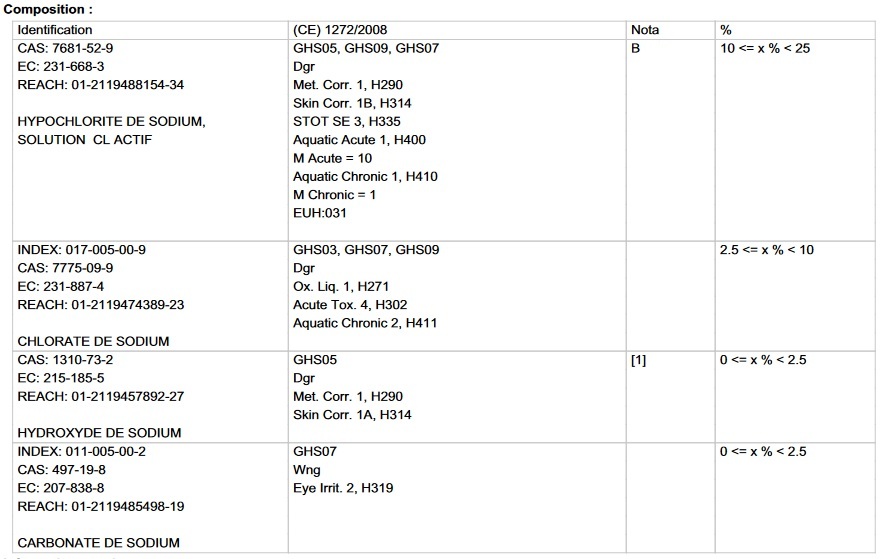
and so I'm confused... should I base it on 13%? 10%? 12.5%? according to these details...
so as not to have an excess of acetone
I am currently preparing my notes for future synthesis and I have a question about the choroform synthesis
I have this product:
it says 13% so I'm doing my calculations... (if i was good)
right?
but then I see in the datasheet these details:
and so I'm confused... should I base it on 13%? 10%? 12.5%? according to these details...
so as not to have an excess of acetone
