G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,426
- Solutions
- 3
- Reaction score
- 2,418
- Points
- 113
- Deals
- 1
Introduction
Some compounds are capable of sublimation, which is the direct phase change from solid to gas. Solid carbon dioxide is an example of a substance that sublimes readily at atmospheric pressure, as a chunk of dry ice will not melt, but will seem to "disappear" as it turns directly into carbon dioxide gas. Sublimation is an analogous process to boiling, as it occurs when a compound's vapor pressure equals its applied pressure (often the atmospheric pressure). The difference is that sublimation involves a solid's vapor pressure instead of a liquid's. Most solids do not have an appreciable vapor pressure at easily accessible temperatures, and for this reason, the ability to sublime is uncommon. Compounds that are capable of sublimation tend to be those with weak intermolecular forces in the solid state. These include compounds with symmetrical or spherical structures. Examples of compounds that can be sublimed are represented below: DMT; magnesium 4-hydroxybutyrate; TMA and so on.
This method is used for purification of substances. The advantages of these processes include: a relatively high equilibrium separation coefficient; the possibility, in the case of using gas mixtures, to exclude the evaporation of solvents (as opposed to absorption and rectification); lower operating temperature than while distillation; ease of control over the coating process; the ability to obtain target products immediately in a commercial form (dispersed particles, single crystals, solid films), high-purity materials, compositions of non-fusion components (whiskers from non-metals in a metallic matrix), thin and ultrafine powders of metals, their oxides.
This method is used for purification of substances. The advantages of these processes include: a relatively high equilibrium separation coefficient; the possibility, in the case of using gas mixtures, to exclude the evaporation of solvents (as opposed to absorption and rectification); lower operating temperature than while distillation; ease of control over the coating process; the ability to obtain target products immediately in a commercial form (dispersed particles, single crystals, solid films), high-purity materials, compositions of non-fusion components (whiskers from non-metals in a metallic matrix), thin and ultrafine powders of metals, their oxides.
Compounds that can be vacuuming sublimed
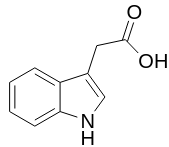
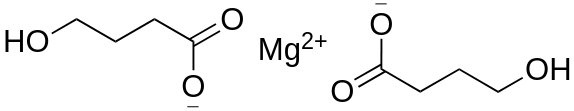
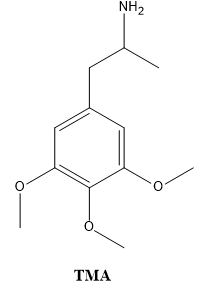
As relatively few solids are capable of sublimation, the process can be an excellent purification method when a volatile solid is contaminated with non-volatile impurities. The impure solid is heated in the bottom of a vessel in close proximity to a cold surface, called a "cold finger" (Fig. 1). As the volatile solid sublimes, it is deposited on the surface of the cold finger (where it can later be recovered), and is thus separated from the non-volatile substance left in the vessel. The process, however, is not particularly efficient at separating volatile solids from one another.
Of the solids with appreciable vapor pressures at room temperature, many still require rather high temperatures to actively sublime (when their vapor pressure equals the atmospheric pressure of nearly 760 mm Hg). If these solids are heated to their sublimation points under atmospheric pressure, some will char and decompose during the process. For this reason, it is very common to perform sublimation under a reduced pressure (vacuum sublimation). Analogous to vacuum distillation in which liquid boils when its vapor pressure equals the reduced pressure in the apparatus, in vacuum sublimation solid sublimes when its vapor pressure equals the reduced pressure in the apparatus. In vacuum distillation, reducing the pressure allows for liquids to boil at a lower temperature. Similarly, reducing the pressure in vacuum sublimation allows for solids to sublime at a lower temperature, one which avoids decomposition.
Step-by-step sublimation procedures
Under Atmospheric Pressure
The sublimation shown in this section shows purification of 0.29 g of ferrocene, which grew in long needles on the bottom and top of the Petri dishes (90% recovery).
1. Spread the crude, dry solid to be sublimed in a thin layer on a "bottom" Petri dish (Fig.2 a). If chunky, first crush with a mortar and pestle. Determine the empty mass of the top Petri dish. It is important that the solid to be purified is dry: if the sample is wet with solvent, condensation may form on the top Petri dish during the sublimation. In the beginning stages of the sublimation, small amounts of condensation can be wiped off the top Petri dish with a paper towel. However, too much condensation may wash crystals off from the top dish.
2. Cover the bottom Petri dish with the top dish and set atop a wire mesh on a hotplate in the fume hood (Fig.2 b) set to the appropriate temperature (depending on the sublimation point of the compound of interest, perhaps medium low). The wire mesh helps dissipate the heat evenly to the dish and minimizes charring.
3. Place a large 600 ml beaker filled with ice water atop the Petri dish (Fig.2 c).
2. Cover the bottom Petri dish with the top dish and set atop a wire mesh on a hotplate in the fume hood (Fig.2 b) set to the appropriate temperature (depending on the sublimation point of the compound of interest, perhaps medium low). The wire mesh helps dissipate the heat evenly to the dish and minimizes charring.
3. Place a large 600 ml beaker filled with ice water atop the Petri dish (Fig.2 c).
4. Over time, the sample will sublime and collect on the upper Petri dish (Fig. 3). Monitor the sublimation as compounds may char during the process (if it starts to blacken, turn down the heat). Continue the sublimation until it appears as if little (or no) solid remains on the bottom Petri dish. It is very common for crystals to also grow along the sides of the bottom dish.
5. Delicately remove the Petri dishes from the hotplate using cotton gloves (Fig.4 a) or a silicone hot hand protector. Jostling the dishes will cause sublimated crystals to fall from the top Petri dish.
Safety note: Allow the two dishes to cool intact on a ceramic tile in the fume hood (Fig.4 b). Do not remove the top Petri dish right away, or noxious fumes may escape.
6. The crystals on the top Petri dish are purified and should be retained (and their mass determined). Sometimes material on the bottom dish may also be saved if it appears crystalline (signifying it underwent a sublimation process) and doesn't appear contaminated with char (Fig.4 c).
5. Delicately remove the Petri dishes from the hotplate using cotton gloves (Fig.4 a) or a silicone hot hand protector. Jostling the dishes will cause sublimated crystals to fall from the top Petri dish.
Safety note: Allow the two dishes to cool intact on a ceramic tile in the fume hood (Fig.4 b). Do not remove the top Petri dish right away, or noxious fumes may escape.
6. The crystals on the top Petri dish are purified and should be retained (and their mass determined). Sometimes material on the bottom dish may also be saved if it appears crystalline (signifying it underwent a sublimation process) and doesn't appear contaminated with char (Fig.4 c).
Under reduced pressure (vacuum sublimation)
The sublimation in this section shows purification of camphor on two scales 2.28 g (large scale, 77% recovery), and roughly 0.2 g (small scale).
1. If the solid to be sublimated is chunky, first crush with a mortar and pestle (Fig.5 a). Then place the crude, dry solid in the bottom of the sublimation apparatus (Fig.5 b). It is important that the solid is dry: if the sample is wet with solvent, condensation may form on the cold finger during the sublimation. Too much condensation may wash crystals off the cold finger.
2. Secure the apparatus to a ring stand or latticework (Fig.5 c and d). For small scales, support the apparatus with a platform (Fig.5 d). A large scale sublimator is shown in Fig.5 c.
3. Lightly grease the joint that connects the two pieces of sublimation glassware. Grease can be easily applied with a syringe full of grease. If using ground glass, lightly grease the joint near the end that will not be in contact with the sample (Fig.5 d).
4. Insert the top piece of the sublimation apparatus (cold finger), and twist the two pieces of glassware together to spread the grease in the joint. When using ground glass, the grease should cause the bottom half of the joint to become transparent all the way around (Fig.6 a). If the entire joint becomes transparent, too much grease has been used and some should be wiped off.
5. Use thick-walled rubber tubing (clear hose in Fig.6 a) to connect the apparatus to a vacuum source (vacuum line or water aspirator). Apply the vacuum. The setup should not hiss or there is a leak in the system.
6. Prepare the cold finger:
5. Use thick-walled rubber tubing (clear hose in Fig.6 a) to connect the apparatus to a vacuum source (vacuum line or water aspirator). Apply the vacuum. The setup should not hiss or there is a leak in the system.
6. Prepare the cold finger:
a) If the cold finger has a condenser, connect water hoses such that the lower arm connects to the water spigot and the upper arm drains to the sink (tan hoses in Fig.6 a). Begin circulating water through the condenser.
b) If the cold finger is an empty tube, fill the cold finger to the brim with ice, then pour in enough water to fill the finger about three-quarters of the way (Fig.6 b). In some cases, the cold finger could be filled with dry ice and acetone.
c) It is proper technique to apply the vacuum before cooling the finger to prevent water condensation from forming, which could wash crystals off the cold finger.
7. Heat the solid with a heat gun (Fig.6 c) or Bunsen burner, beginning slowly with a back and forth motion and low heat. It is not recommended using a sand bath or heating mantle for sublimation, as heating is often too slow and can only direct heat to the bottom of the apparatus, not the sides. Increase the rate of heating if the sublimation does not begin within a few minutes.
8. Over a short amount of time, solid should begin to deposit on the cold finger. It will undoubtedly also deposit on the outsides of the glassware (Fig.6 d). Solid can be coaxed away from the outside of the glassware and toward the cold finger by waving the heat gun or burner periodically up the sides of the glass.
8. Over a short amount of time, solid should begin to deposit on the cold finger. It will undoubtedly also deposit on the outsides of the glassware (Fig.6 d). Solid can be coaxed away from the outside of the glassware and toward the cold finger by waving the heat gun or burner periodically up the sides of the glass.
9. Continue the sublimation until all the volatile substance is transferred from the bottom piece of glassware to the cold finger (Fig. 7). If the compound begins to darken, decrease the rate of heating to prevent decomposition.
10. Remove the coolant from the cold finger:
10. Remove the coolant from the cold finger:
a) If a condenser was used, turn off the circulating water and remove the water hoses from the apparatus (carefully, without making a large mess).
b) If an ice water coolant was used, scoop out the ice if possible, and remove the water by pipette (or for large scales with a turkey baster, Fig.7 a).
11. Allow the system to come to room temperature.
12. Delicately reinstate air pressure to the apparatus (Fig.7 b), noting that an abrupt opening of the system will cause air to violently enter the apparatus and will likely cause crystals to dislodge from the cold finger.
13. Delicately remove the emptied cold finger from the apparatus, and scrape the sublimed crystals onto a watch glass (Fig.7 c). Alternatively, rinse the crystals from the cold finger with solvent through a funnel and into a round bottomed flask (Fig.7 d), to later remove the solvent using a rotary evaporator.
13. Delicately remove the emptied cold finger from the apparatus, and scrape the sublimed crystals onto a watch glass (Fig.7 c). Alternatively, rinse the crystals from the cold finger with solvent through a funnel and into a round bottomed flask (Fig.7 d), to later remove the solvent using a rotary evaporator.
Last edited by a moderator:
