WillD
Expert
- Joined
- Jul 19, 2021
- Messages
- 685
- Reaction score
- 969
- Points
- 93
Synthesis of 2-allyoxyphenol:
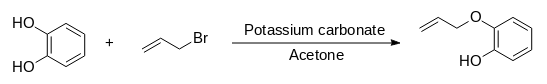
1. Catechol (20 g, 182 mmol), potassium carbonate (25.2 g, 182 mmol) and 100ml of acetone were cooled in an ice bath
2. Allyl bromide (22.0 g, 182 mmol) was added dropwise and the mixture was heated under reflux for 4 h.
3. The resulting mixture was left to cool, and the solid material was removed by filtration.
4. The volatile components of the mother liquor were removed using a rotary evaporator leaving an orange residue.
5. The residue was dissolved in diethyl ether (60 ml) and hydrochloric acid (40 ml, 1.6 M) was added.
6. The aqueous layer was removed, and the organic layer was washed with water (4x40 ml).
7. The organic extracts were dried over anhydrous sodium sulphate and decanted.
8. The solvent was removed using a rotary evaporator to produce a yellow/orange liquid. Yield: 16.6 g.
Synthesis of 4-allycatechol (Route 1):
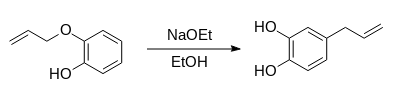
1. 2-Allyloxyphenol (7.5 g) and sodium ethoxide (3.5 g, 51 mmol) were dissolved in anhydrous ethanol (25 ml) and heated under reflux.
2. Additional sodium ethoxide (2.0 g, 29 mmol) was added to the reaction mixture every 24 h.
3. The solution was heated under reflux for a total of 96 h and then left to cool.
4. The resultant mixture was dissolved in hydrochloric acid (20 mL, 3.2 M) and the product was extracted with dichloromethane (3x20 ml).
5. The organic extracts were washed with water (20 ml), dried over anhydrous sodium sulphate and decanted.
6. The solvent was removed using a rotary evaporator to produce a brown liquid.Yield: 6.6 g.
Synthesis of 4-allycatechol (Route 2):
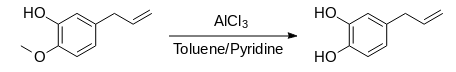
1. Eugenol (8.0 g), aluminium chloride (8.6 g, 64 mmol) and 250 ml of toluene were cooled in an ice bath.
2. Pyridine (18.5 ml, 230 mmol) was added dropwise and the mixture was heated under reflux for 5 h.
3. The resulting mixture was left to cool, and the clear, yellow organic layer was decanted.
4. The remaining solid was dissolved in hydrochloric acid (300 mL, 6.4 M) and extracted with diethyl ether (3x100 ml).
5. The organic extracts were washed with water (3 x100 mL), dried over anhydrous sodium sulphate, decanted and the solvent was removed using a rotary evaporator to produce a black liquid. Yield: 6.4 g.
Synthesis of safrole:
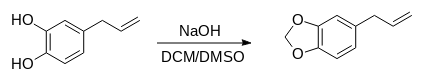
1. A solution containing dichloromethane (5.0 mL, 78 mmol) and 50 mL of dimethyl sulfoxide were heated at 120–130 *C.
2. Sodium hydroxide (2.5 g, 63 mmol) was added to the solution
3. 4-Allylcatechol (4.0 g) was dissolved in dimethylsulfoxide (10 mL) and added dropwise to the mixture, which was heated at 120–130 *C for 45 min.
4. The resulting mixture was decanted and water (50 mL) was added and left to cool.
5. The resulting solution was extracted with diethyl ether (3x25 mL) and the organic layer was washed with water (3x25 ml).
6. The organic extracts were dried over anhydrous sodium sulphate, decanted and the solvent was removed using a rotary evaporator to produce brown liquid.
Route 1 Yield: 3.4 g.
Route 2 Yield: 3.7 g.
1. Catechol (20 g, 182 mmol), potassium carbonate (25.2 g, 182 mmol) and 100ml of acetone were cooled in an ice bath
2. Allyl bromide (22.0 g, 182 mmol) was added dropwise and the mixture was heated under reflux for 4 h.
3. The resulting mixture was left to cool, and the solid material was removed by filtration.
4. The volatile components of the mother liquor were removed using a rotary evaporator leaving an orange residue.
5. The residue was dissolved in diethyl ether (60 ml) and hydrochloric acid (40 ml, 1.6 M) was added.
6. The aqueous layer was removed, and the organic layer was washed with water (4x40 ml).
7. The organic extracts were dried over anhydrous sodium sulphate and decanted.
8. The solvent was removed using a rotary evaporator to produce a yellow/orange liquid. Yield: 16.6 g.
Synthesis of 4-allycatechol (Route 1):
1. 2-Allyloxyphenol (7.5 g) and sodium ethoxide (3.5 g, 51 mmol) were dissolved in anhydrous ethanol (25 ml) and heated under reflux.
2. Additional sodium ethoxide (2.0 g, 29 mmol) was added to the reaction mixture every 24 h.
3. The solution was heated under reflux for a total of 96 h and then left to cool.
4. The resultant mixture was dissolved in hydrochloric acid (20 mL, 3.2 M) and the product was extracted with dichloromethane (3x20 ml).
5. The organic extracts were washed with water (20 ml), dried over anhydrous sodium sulphate and decanted.
6. The solvent was removed using a rotary evaporator to produce a brown liquid.Yield: 6.6 g.
Synthesis of 4-allycatechol (Route 2):
1. Eugenol (8.0 g), aluminium chloride (8.6 g, 64 mmol) and 250 ml of toluene were cooled in an ice bath.
2. Pyridine (18.5 ml, 230 mmol) was added dropwise and the mixture was heated under reflux for 5 h.
3. The resulting mixture was left to cool, and the clear, yellow organic layer was decanted.
4. The remaining solid was dissolved in hydrochloric acid (300 mL, 6.4 M) and extracted with diethyl ether (3x100 ml).
5. The organic extracts were washed with water (3 x100 mL), dried over anhydrous sodium sulphate, decanted and the solvent was removed using a rotary evaporator to produce a black liquid. Yield: 6.4 g.
Synthesis of safrole:
1. A solution containing dichloromethane (5.0 mL, 78 mmol) and 50 mL of dimethyl sulfoxide were heated at 120–130 *C.
2. Sodium hydroxide (2.5 g, 63 mmol) was added to the solution
3. 4-Allylcatechol (4.0 g) was dissolved in dimethylsulfoxide (10 mL) and added dropwise to the mixture, which was heated at 120–130 *C for 45 min.
4. The resulting mixture was decanted and water (50 mL) was added and left to cool.
5. The resulting solution was extracted with diethyl ether (3x25 mL) and the organic layer was washed with water (3x25 ml).
6. The organic extracts were dried over anhydrous sodium sulphate, decanted and the solvent was removed using a rotary evaporator to produce brown liquid.
Route 1 Yield: 3.4 g.
Route 2 Yield: 3.7 g.
Last edited by a moderator:
