- Joined
- May 9, 2023
- Messages
- 9
- Reaction score
- 6
- Points
- 3
Has anybody ever had any luck with this process? I’m confused about the part where it says to add two drops of concentrated sulfuric acid to the ephedrine/acetic acid mixture but it also says to make sure no water is in the reaction mixture. Doesn’t concentrated sulfuric acid contain water? Also he says you could use palladium plated metal but what metal could you use that won’t dissolve in the acid?
___________________________________________
Instruction part from chapter 2 “The Fester Formula”of Advanced Techniques of Clandestine Psychedelic & Amphetamine Manufacture
Now one gram of ephedrine, pseudoephedrine or PPA hy- drochloride is put into a large test tube, along with 5-7 ml of glacial acetic acid. The bottom of the test tube is placed into a pot of hot water, and when the ephedrine hydrochloride, or whatever, is about all dissolved, a few drops of concentrated sulfuric acid are added. Mix it all in, and loosely stopper the end of the test tube with a cork to prevent steam from entering. Heat the hot water bath to just about boiling, and use this hot water bath to heat the test tube and its contents for a few hours. This forms the acetic acid ester of the ephedrine, pseudoephed- rine or PPA, used in the reaction.
The solution should appear clear and water-like, and com- pletely homogenous. After heating, the reaction mixture can be kept stoppered as is with no harm for at least a few days, but it's best to use it immediately after it's cooked and cooled.
This reaction to form the acetic acid ester is a typical ester forming reaction, and the usual rules apply. Water must be kept out of the reaction mixture, as its presence greatly reduces the yield. As a consequence, only crystalline ephedrine, pseudo- ephedrine or PPA hydrochloride can be fed into the process. A concentrated water extract won't do. An excess of acetic acid pushes the equilibrium toward making more ester. As a result, 7 or more ml of acetic acid is preferable to 5 ml. Only glacial acetic acid can be used, as diluted acetic acid is full of water. It would be best to reflux the ester forming mixture, but the simple procedure given here, heating to about the boiling point of water with precaution to keep steam from getting into the test tube, works good enough to give satisfying results.
.Nextmixupasolutionof5mlofconcentratedsulfuric acid in 100 ml of water. Take a 250 ml beaker, place it on a mag-
I, ' 'I I•
iI
Advanced Techniques of Clandestine Psychedelic & Amphetamine Manufacture
18
netic stirrer. Clip a well scrubbed Kling-Tite Naturalamb rub- ber in one side of the beaker, and put a piece of lead about vi- inch in diameter, and a few inches long, inside the rubber. On the opposite side of the beaker, stand up a one ounce ingot of palladium Using alligator clips, make contact with the ingot, and with the piece o f lead. Next pour most of the dilute sulfuric acid solution into the beaker. Save enough that some can be poured into the rubber so that the solution levels are about equal inside the rubber and the beaker at large. The ingot of palladium should be almost completely immersed. The alligator clip should be up out of the solution and there should be enough space left to add the ester reaction mixture from the test tube to the beaker without causing the solution level to reach the alligator clip. See the drawing below.
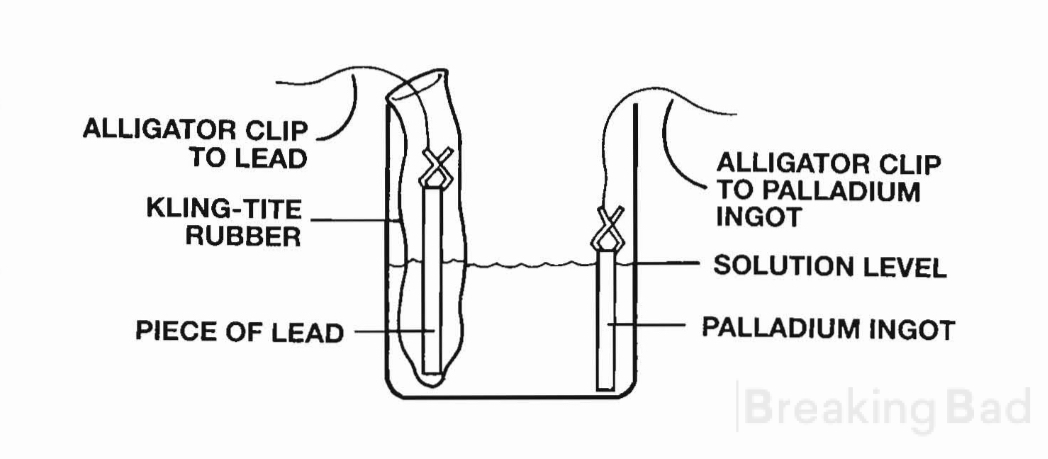
The choice of a 250 ml beaker here is based solely upon having room inside the beaker to put a standard size magnetic stir bar along with the two electrodes and rubber. A 100 ml beaker would no doubt be superior, as the one ounce palladium ingot would be considerably larger in relation to the catholyte
Chapter Two The Fester Formula
19
volume in a 100 m1 beaker. The ester of ephedrine, pseudo- ephedrine or PPA would also be considerably more concen- trated in a 100 m1 beaker, allowing for more efficient electric reduction. In a 250 m1 beaker, the ester reaction mixture will be diluted over 10 times by the catholyte whereas in a 100 m1 beaker, the dilution will be more like five times. A perfectly usable magnetic stir bar can be made by cutting a section of bar magnet and coating it with a few coats of tough paint. In this way, a suitable size stir bar for the smaller beaker can easily be made.
A glass beaker isn't the only reaction vessel which can be used. The only requirements are that it be non-conductive so that the cell doesn't short out, and it must also be inert to the dilute acetic and sulfuric acid used in the process. A measuring cup with a pour lip would be a quite good substitute, and a drink tumbler would also be serviceable.
The lead anode shown in the drawing can be replaced with other materials as well. The only function of the anode is to pump current into the solution. It doesn't take part in the reac- tion in any other way. Suitable replacements for lead would be graphite rod obtained at the welding supply shop or dissected out of a dry cell battery. One could also use platinum metal. Unsuitable choices for anode materials include iron and steel, copper and brass, and aluminum. All of these metals will dis- solve in dilute sulfuric acid when made anodic. The exact size or shape ofthe anode isn't particularly important. So long as it has dimensions about that shown in the drawing, it will work fine. As stated earlier, its only function is to pump current into the solution.
The rubber serves two purposes. It first of all keeps the ephedrine, pseudoephedrine or PPA from coming into contact with the anode. These substances will oxidize at the anode, resulting in cleavage producing benzaldehyde, methylamine or acetaldehyde. This makes one wonder whether the process could be run backwards with electric reduction. Methamphet- amine will oxidize at the anode to form a tar which clings to the anode surface. See Chern. Pharm. Bull., volume 25, pp. 1619-22 (1980) for more on this subject. The rubber also serves to keep the oxygen which is generated at the anode surface from leaking into the solution by the cathode. This would interfere with the hydrogenation taking place there.
The surface of the palladium ingot should be lightly sanded prior to use. This increases its surface area a little, and exposes fresh clean metal. The piece of lead should be scrubbed free of grease and dirt. The wire lead to the palladium ingot can be clipped to the side of the beaker with a clothes pin or paper clip, to prevent the ingot from falling over during the course of the reaction. A DC current meter (amp meter) should be put in line on the wiring. A perfectly good one can be had at Radio Shack for about $50. Note that the model I have was made in China, and the instructions for how to wire it in to measure current were wrong. You'll figure it out, I'm sure.
First the wires are hooked up so that the palladium ingot is connected to the positive pole of the DC transformer, and the piece of lead to the negative. The typical one ounce ingot will have a face with an area of about 6 square centimeters im- mersed in the solution, and about one square centimeter up out of the solution. Only count the area on the side facing the lead piece. The back side doesn't count because current can't reach it. With this typical size ingot apply about 2 amps for one or two minutes. Oxygen will bubble freely from the ingot, and hydrogen from the piece of lead. Blackening will be noted on the edges of the ingot, where the current is most intense, and a lighter discoloration on the flat face of the ingot. This pre- treatment is called "anodizing." It has been found that anodiz- ing increases the ability of the palladium ingot to absorb hy- drogen when the wiring is turned around, and the ingot is made the cathode.
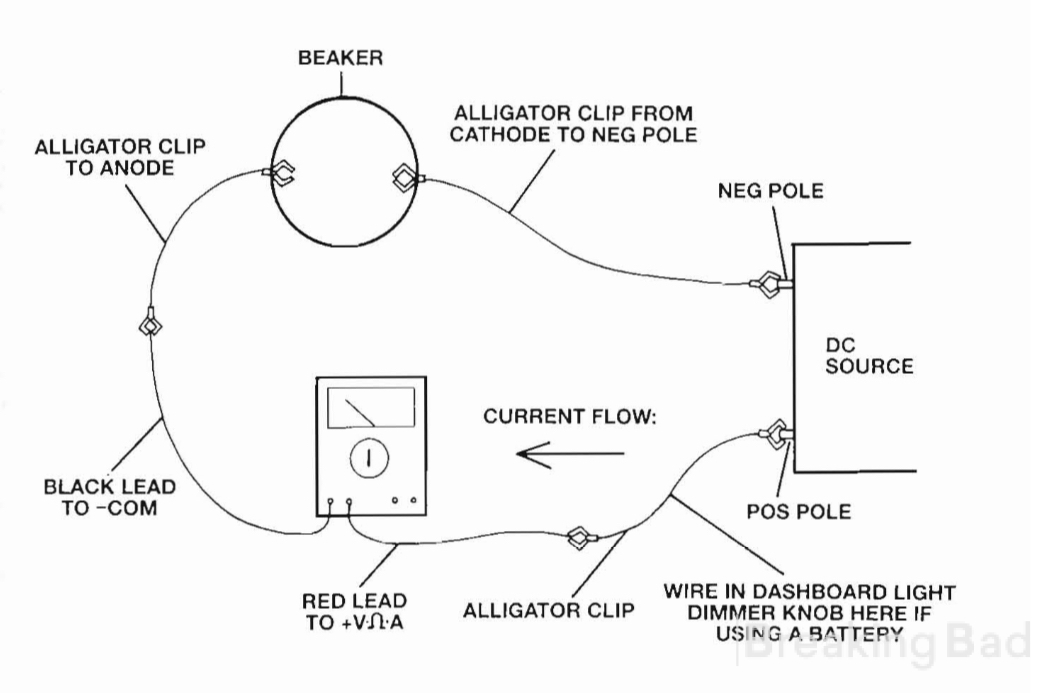
Next, redo the wiring so that the palladium ingot is attached to the negative pole of the DC transformer, and the piece of lead to the positive. Tum back on the juice, and for this typical size ingot, run between one and two amps of current for about 20 minutes. At first, the amount of hydrogen generated at the palladium ingot will appear small, because it absorbs the hy- drogen so well. After about 5 minutes of current passage, the whole surface of the ingot will freely bubble off hydrogen.
An alternative to the use of sulfuric acid electrolyte is to use 2% HCI solution. In this case, the ingot is first hooked up as anode, and a current of one or two amps is applied for a minute or two. The surface layer o f the ingot will dissolve as a reddish brown solution of palladium chloride. Then the palladium ingot is made the cathode and about 50 milliamps per square em of face applied for about 10 to 20 minutes . Most o f the dissolved PdClz will electroplate onto the surface of the ingot. The sur- face treatment is called "palladized palladium." Now "anodize" in dilute sulfuric acid solution as in the previous example. Next, return the ingot to the 2% Hel solution and charge up the ingot with hydrogen for about 20 minutes as in the previous example . The electrocatalytic hydrogenation o f the acetic acid ester of ephedrine, or whatever, is then done in this solution, just like in the example which follows. A lead anode can't be used in this variation, as it would dissolve. Other than being more complicated, this variation is probably superior to using dilute sulfuric acid, as this acid tends to poison the catalytic
'I, property of the palladium surface over time.
After the 20 minute charging with hydrogen, begin magnetic
stirring of the solution, then pour in the ester reaction mixture from the large test tube. Adjust the current flow to between 35- 50 milliamps per square centimeter o f the ingot face. I f one has 6 square centimeters of the ingot facing the lead anode actually immersed in the solution, a current of between 200-300 milli- amps is called for.
This will result in some gassing off of hydrogen from the edges of the ingot, but over the rest of the surface of the ingot the hydrogen formed will react before it bubbles off. The lead anode will form a brown layer of lead oxide, and not dissolve at all in the sulfuric acid solution. Some surface particles will be kicked off the lead when it's first charging, but they don't make it through the rubber. The lead anode can be replaced with a piece of platinum i f one desires, but lead is a lot cheaper.
Keep an eye on the current meter, and make sure that the current flow stays in the 200-300 milliamp (.2-.3 amp) range for the size ingot given in this example. Allowing too much current to flow will cause the ingot surface to be covered by hydrogen bubbles, and the solution won't be able to come into free contact with the metal surface. Turning the current down too low may result in no hydrogen being formed at the palla- dium surface.
The best and most convenient DC electric power source is a rectifier such as those commonly used by electroplaters to do lab scale electroplating testing and experimentation. Such rectifiers cost about $500-600 from suppliers of electroplating equipment. Using such a device, the current flow is easily controlled by turning up or down the voltage output of the rectifier. The higher the voltage output, the more current is passed through the solution. E=IR.
The next best current source is a 12 volt car battery with its voltage output modulated by hooking into the wiring to the beaker a dashboard light control knob. This dashboard light control will cost a few bucks at the auto parts store, and will function in this electric cell just as on the dashboard. Tum the knob up, as you would to brighten the dashboard light, and the voltage is increased and more current will pass through the solution.
A toy train transformer may also work, but beware of a thing called "AC ripple" found with such cheap power sources. This is where AC current is superimposed upon the DC current. Generally it will produce a "picket fence" output visible on an oscilloscope. So long as the spikes all run in the desired direc- tion, I think that it will work OK. If, on the other hand, the AC ripple causes the palladium ingot to oscillate between anodic and cathodic, you're in for trouble.
When about 3000 coulombs have passed through the solu- tion, the process can be considered to be complete for a one gram batch. A coulomb is one arnp-second, so let's use the 300 milliamp current flow to calculate reaction time. 3000 amp- seconds divided by .3 amp =10,000 seconds, or 2 hours 45 minutes.
Three thousand coulombs per gram o f feed material has been found to give good yields of a fine product, but by no means assume this number to be the optimal. It may well be that greater yields would be obtained by passing more current. It
may also be that pseudoephedrine and PPA differ from ephed- rine in their ease of electrocatalytic hydrogenation, and again require the passage of more current. I don't think that any harm can be done by passing too much current, within reasonable limits, so by all means experiment with the amount of current passed.
During the course of the reduction, the color of the reaction mixture slowly changes from its initially clear color to slightly tinted with yellow. It's not known if this color change is due to some of the Kling-Tite rubber soaking out to form a tea, or if
,II it is the result of the reaction. In any case, this is a remarkably clean reaction.
When the desired amount ofcurrent has passed, the work-up and isolation of the product is very simple. The Kling-Tite rubber is removed from the beaker. After pulling out the lead or platinum anode, the rubber is flushed down the toilet. The anode can be reused over and over. Then the palladium cathode is removed, and rinsed off. It too can be reused an innumerable number of times. The process of "anodizing" the palladium will have to be repeated prior to each run. Some fresh metal may have to be exposed on occasion by light sanding of the metal surface. An ingot of palladium should last for a lifetime.
The reaction mixture should be poured into a sep funnel. and approximately 20% solution o f NaOH (lye) in water should be added with shaking until the mixture is strongly (13+) alkaline to pH paper. Then extract with one or two portions of toluene. Fifty to one hundred ml of toluene is more than enough to extract one gram of product. The toluene extracts are next bubbled with dry Hel to get the crystalline hydrochloride product. After filtering and rinsing them off with some fresh toluene, they are spread out to dry. The most pleasantly sur- prising fmding is that crank produced by this method doesn't give one the body and soul wrenching hangovers so typical of the product made by the HI and red phosphorus method. This
process is a highly desirable way to keep one's own party rocking and rolling.
If one should wish to produce more than a gram or so at a time, a larger palladium catalytic cathode should be used. Linking together more ingots of palladium would get pretty expensive, so a more economical alternative will be detailed. That alternative is electroplating some copper or brass screen with a thick coating of palladium.
The simplest way to get this section of screen electroplated with palladium is to go to the yellow pages, look under electro- platers, and fmd one who electroplates palladium. Ask for a plate build-up of several thousandths of an inch thickness, so that enough palladium is deposited to last for a while.
This palladium plated screen would then be used exactly like an ingot o f palladium. First it must be "anodized," then charged up with hydrogen in exactly the same way. The sole difference is that the greater surface area of the screen facing the rubber ensconced anode requires a correspondingly greater amount of current be passed. Then during the course of the reduction, again 50 milliamps per square centimeter o f surface area facing the anode is used. The total of 3000 or so coulombs per gram of feed material doesn't change by increasing the size of the catalytic cathode.
An alternative to sending a section o f screen out to be plated is to plate it yourself. One starts with an ingot of palladium, and anodically dissolves a portion of it to form a PdCh solution. Follow the directions for doing this in the PdClz chapter in this book. The procedure given here using a Kling-Tite rubber as a shield for the negative pole ofthe circuit works very well in my experience. The concentration of PdClz in solution is found by weighing the palladium anode as it dissolves. The amount dissolved times 1.7 is the amount ofPdClz in solution.
___________________________________________
Instruction part from chapter 2 “The Fester Formula”of Advanced Techniques of Clandestine Psychedelic & Amphetamine Manufacture
Now one gram of ephedrine, pseudoephedrine or PPA hy- drochloride is put into a large test tube, along with 5-7 ml of glacial acetic acid. The bottom of the test tube is placed into a pot of hot water, and when the ephedrine hydrochloride, or whatever, is about all dissolved, a few drops of concentrated sulfuric acid are added. Mix it all in, and loosely stopper the end of the test tube with a cork to prevent steam from entering. Heat the hot water bath to just about boiling, and use this hot water bath to heat the test tube and its contents for a few hours. This forms the acetic acid ester of the ephedrine, pseudoephed- rine or PPA, used in the reaction.
The solution should appear clear and water-like, and com- pletely homogenous. After heating, the reaction mixture can be kept stoppered as is with no harm for at least a few days, but it's best to use it immediately after it's cooked and cooled.
This reaction to form the acetic acid ester is a typical ester forming reaction, and the usual rules apply. Water must be kept out of the reaction mixture, as its presence greatly reduces the yield. As a consequence, only crystalline ephedrine, pseudo- ephedrine or PPA hydrochloride can be fed into the process. A concentrated water extract won't do. An excess of acetic acid pushes the equilibrium toward making more ester. As a result, 7 or more ml of acetic acid is preferable to 5 ml. Only glacial acetic acid can be used, as diluted acetic acid is full of water. It would be best to reflux the ester forming mixture, but the simple procedure given here, heating to about the boiling point of water with precaution to keep steam from getting into the test tube, works good enough to give satisfying results.
.Nextmixupasolutionof5mlofconcentratedsulfuric acid in 100 ml of water. Take a 250 ml beaker, place it on a mag-
I, ' 'I I•
iI
Advanced Techniques of Clandestine Psychedelic & Amphetamine Manufacture
18
netic stirrer. Clip a well scrubbed Kling-Tite Naturalamb rub- ber in one side of the beaker, and put a piece of lead about vi- inch in diameter, and a few inches long, inside the rubber. On the opposite side of the beaker, stand up a one ounce ingot of palladium Using alligator clips, make contact with the ingot, and with the piece o f lead. Next pour most of the dilute sulfuric acid solution into the beaker. Save enough that some can be poured into the rubber so that the solution levels are about equal inside the rubber and the beaker at large. The ingot of palladium should be almost completely immersed. The alligator clip should be up out of the solution and there should be enough space left to add the ester reaction mixture from the test tube to the beaker without causing the solution level to reach the alligator clip. See the drawing below.
The choice of a 250 ml beaker here is based solely upon having room inside the beaker to put a standard size magnetic stir bar along with the two electrodes and rubber. A 100 ml beaker would no doubt be superior, as the one ounce palladium ingot would be considerably larger in relation to the catholyte
Chapter Two The Fester Formula
19
volume in a 100 m1 beaker. The ester of ephedrine, pseudo- ephedrine or PPA would also be considerably more concen- trated in a 100 m1 beaker, allowing for more efficient electric reduction. In a 250 m1 beaker, the ester reaction mixture will be diluted over 10 times by the catholyte whereas in a 100 m1 beaker, the dilution will be more like five times. A perfectly usable magnetic stir bar can be made by cutting a section of bar magnet and coating it with a few coats of tough paint. In this way, a suitable size stir bar for the smaller beaker can easily be made.
A glass beaker isn't the only reaction vessel which can be used. The only requirements are that it be non-conductive so that the cell doesn't short out, and it must also be inert to the dilute acetic and sulfuric acid used in the process. A measuring cup with a pour lip would be a quite good substitute, and a drink tumbler would also be serviceable.
The lead anode shown in the drawing can be replaced with other materials as well. The only function of the anode is to pump current into the solution. It doesn't take part in the reac- tion in any other way. Suitable replacements for lead would be graphite rod obtained at the welding supply shop or dissected out of a dry cell battery. One could also use platinum metal. Unsuitable choices for anode materials include iron and steel, copper and brass, and aluminum. All of these metals will dis- solve in dilute sulfuric acid when made anodic. The exact size or shape ofthe anode isn't particularly important. So long as it has dimensions about that shown in the drawing, it will work fine. As stated earlier, its only function is to pump current into the solution.
The rubber serves two purposes. It first of all keeps the ephedrine, pseudoephedrine or PPA from coming into contact with the anode. These substances will oxidize at the anode, resulting in cleavage producing benzaldehyde, methylamine or acetaldehyde. This makes one wonder whether the process could be run backwards with electric reduction. Methamphet- amine will oxidize at the anode to form a tar which clings to the anode surface. See Chern. Pharm. Bull., volume 25, pp. 1619-22 (1980) for more on this subject. The rubber also serves to keep the oxygen which is generated at the anode surface from leaking into the solution by the cathode. This would interfere with the hydrogenation taking place there.
The surface of the palladium ingot should be lightly sanded prior to use. This increases its surface area a little, and exposes fresh clean metal. The piece of lead should be scrubbed free of grease and dirt. The wire lead to the palladium ingot can be clipped to the side of the beaker with a clothes pin or paper clip, to prevent the ingot from falling over during the course of the reaction. A DC current meter (amp meter) should be put in line on the wiring. A perfectly good one can be had at Radio Shack for about $50. Note that the model I have was made in China, and the instructions for how to wire it in to measure current were wrong. You'll figure it out, I'm sure.
First the wires are hooked up so that the palladium ingot is connected to the positive pole of the DC transformer, and the piece of lead to the negative. The typical one ounce ingot will have a face with an area of about 6 square centimeters im- mersed in the solution, and about one square centimeter up out of the solution. Only count the area on the side facing the lead piece. The back side doesn't count because current can't reach it. With this typical size ingot apply about 2 amps for one or two minutes. Oxygen will bubble freely from the ingot, and hydrogen from the piece of lead. Blackening will be noted on the edges of the ingot, where the current is most intense, and a lighter discoloration on the flat face of the ingot. This pre- treatment is called "anodizing." It has been found that anodiz- ing increases the ability of the palladium ingot to absorb hy- drogen when the wiring is turned around, and the ingot is made the cathode.
Next, redo the wiring so that the palladium ingot is attached to the negative pole of the DC transformer, and the piece of lead to the positive. Tum back on the juice, and for this typical size ingot, run between one and two amps of current for about 20 minutes. At first, the amount of hydrogen generated at the palladium ingot will appear small, because it absorbs the hy- drogen so well. After about 5 minutes of current passage, the whole surface of the ingot will freely bubble off hydrogen.
An alternative to the use of sulfuric acid electrolyte is to use 2% HCI solution. In this case, the ingot is first hooked up as anode, and a current of one or two amps is applied for a minute or two. The surface layer o f the ingot will dissolve as a reddish brown solution of palladium chloride. Then the palladium ingot is made the cathode and about 50 milliamps per square em of face applied for about 10 to 20 minutes . Most o f the dissolved PdClz will electroplate onto the surface of the ingot. The sur- face treatment is called "palladized palladium." Now "anodize" in dilute sulfuric acid solution as in the previous example. Next, return the ingot to the 2% Hel solution and charge up the ingot with hydrogen for about 20 minutes as in the previous example . The electrocatalytic hydrogenation o f the acetic acid ester of ephedrine, or whatever, is then done in this solution, just like in the example which follows. A lead anode can't be used in this variation, as it would dissolve. Other than being more complicated, this variation is probably superior to using dilute sulfuric acid, as this acid tends to poison the catalytic
'I, property of the palladium surface over time.
After the 20 minute charging with hydrogen, begin magnetic
stirring of the solution, then pour in the ester reaction mixture from the large test tube. Adjust the current flow to between 35- 50 milliamps per square centimeter o f the ingot face. I f one has 6 square centimeters of the ingot facing the lead anode actually immersed in the solution, a current of between 200-300 milli- amps is called for.
This will result in some gassing off of hydrogen from the edges of the ingot, but over the rest of the surface of the ingot the hydrogen formed will react before it bubbles off. The lead anode will form a brown layer of lead oxide, and not dissolve at all in the sulfuric acid solution. Some surface particles will be kicked off the lead when it's first charging, but they don't make it through the rubber. The lead anode can be replaced with a piece of platinum i f one desires, but lead is a lot cheaper.
Keep an eye on the current meter, and make sure that the current flow stays in the 200-300 milliamp (.2-.3 amp) range for the size ingot given in this example. Allowing too much current to flow will cause the ingot surface to be covered by hydrogen bubbles, and the solution won't be able to come into free contact with the metal surface. Turning the current down too low may result in no hydrogen being formed at the palla- dium surface.
The best and most convenient DC electric power source is a rectifier such as those commonly used by electroplaters to do lab scale electroplating testing and experimentation. Such rectifiers cost about $500-600 from suppliers of electroplating equipment. Using such a device, the current flow is easily controlled by turning up or down the voltage output of the rectifier. The higher the voltage output, the more current is passed through the solution. E=IR.
The next best current source is a 12 volt car battery with its voltage output modulated by hooking into the wiring to the beaker a dashboard light control knob. This dashboard light control will cost a few bucks at the auto parts store, and will function in this electric cell just as on the dashboard. Tum the knob up, as you would to brighten the dashboard light, and the voltage is increased and more current will pass through the solution.
A toy train transformer may also work, but beware of a thing called "AC ripple" found with such cheap power sources. This is where AC current is superimposed upon the DC current. Generally it will produce a "picket fence" output visible on an oscilloscope. So long as the spikes all run in the desired direc- tion, I think that it will work OK. If, on the other hand, the AC ripple causes the palladium ingot to oscillate between anodic and cathodic, you're in for trouble.
When about 3000 coulombs have passed through the solu- tion, the process can be considered to be complete for a one gram batch. A coulomb is one arnp-second, so let's use the 300 milliamp current flow to calculate reaction time. 3000 amp- seconds divided by .3 amp =10,000 seconds, or 2 hours 45 minutes.
Three thousand coulombs per gram o f feed material has been found to give good yields of a fine product, but by no means assume this number to be the optimal. It may well be that greater yields would be obtained by passing more current. It
may also be that pseudoephedrine and PPA differ from ephed- rine in their ease of electrocatalytic hydrogenation, and again require the passage of more current. I don't think that any harm can be done by passing too much current, within reasonable limits, so by all means experiment with the amount of current passed.
During the course of the reduction, the color of the reaction mixture slowly changes from its initially clear color to slightly tinted with yellow. It's not known if this color change is due to some of the Kling-Tite rubber soaking out to form a tea, or if
,II it is the result of the reaction. In any case, this is a remarkably clean reaction.
When the desired amount ofcurrent has passed, the work-up and isolation of the product is very simple. The Kling-Tite rubber is removed from the beaker. After pulling out the lead or platinum anode, the rubber is flushed down the toilet. The anode can be reused over and over. Then the palladium cathode is removed, and rinsed off. It too can be reused an innumerable number of times. The process of "anodizing" the palladium will have to be repeated prior to each run. Some fresh metal may have to be exposed on occasion by light sanding of the metal surface. An ingot of palladium should last for a lifetime.
The reaction mixture should be poured into a sep funnel. and approximately 20% solution o f NaOH (lye) in water should be added with shaking until the mixture is strongly (13+) alkaline to pH paper. Then extract with one or two portions of toluene. Fifty to one hundred ml of toluene is more than enough to extract one gram of product. The toluene extracts are next bubbled with dry Hel to get the crystalline hydrochloride product. After filtering and rinsing them off with some fresh toluene, they are spread out to dry. The most pleasantly sur- prising fmding is that crank produced by this method doesn't give one the body and soul wrenching hangovers so typical of the product made by the HI and red phosphorus method. This
process is a highly desirable way to keep one's own party rocking and rolling.
If one should wish to produce more than a gram or so at a time, a larger palladium catalytic cathode should be used. Linking together more ingots of palladium would get pretty expensive, so a more economical alternative will be detailed. That alternative is electroplating some copper or brass screen with a thick coating of palladium.
The simplest way to get this section of screen electroplated with palladium is to go to the yellow pages, look under electro- platers, and fmd one who electroplates palladium. Ask for a plate build-up of several thousandths of an inch thickness, so that enough palladium is deposited to last for a while.
This palladium plated screen would then be used exactly like an ingot o f palladium. First it must be "anodized," then charged up with hydrogen in exactly the same way. The sole difference is that the greater surface area of the screen facing the rubber ensconced anode requires a correspondingly greater amount of current be passed. Then during the course of the reduction, again 50 milliamps per square centimeter o f surface area facing the anode is used. The total of 3000 or so coulombs per gram of feed material doesn't change by increasing the size of the catalytic cathode.
An alternative to sending a section o f screen out to be plated is to plate it yourself. One starts with an ingot of palladium, and anodically dissolves a portion of it to form a PdCh solution. Follow the directions for doing this in the PdClz chapter in this book. The procedure given here using a Kling-Tite rubber as a shield for the negative pole ofthe circuit works very well in my experience. The concentration of PdClz in solution is found by weighing the palladium anode as it dissolves. The amount dissolved times 1.7 is the amount ofPdClz in solution.



