G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,599
- Solutions
- 3
- Reaction score
- 2,624
- Points
- 113
- Deals
- 1
Introduction
This is one of the simplest ways to obtain 1-Phenyl-2-propanone (P2P). Diethyl(phenylacetyl)malonate can be bought in some web market quite easy or synthesized by yourself using this method. Also, you can learn other P2P synthetic paths in our forum by following links: Synthesis of P2P from benzaldehyde with MEK, Synthesis of P2P by oxidation of alpha-methylstyrene with Oxone, Phenylacetone (P2P) syntheses via Grignard reagents, Industrial phenylacetone (P2P) production from benzene, Synthesis of P2P from P2NP with NaBH4 via K2CO3/H2O2 system.
Difficulty Rating: 3/10
Difficulty Rating: 3/10
Equipment and glassware:
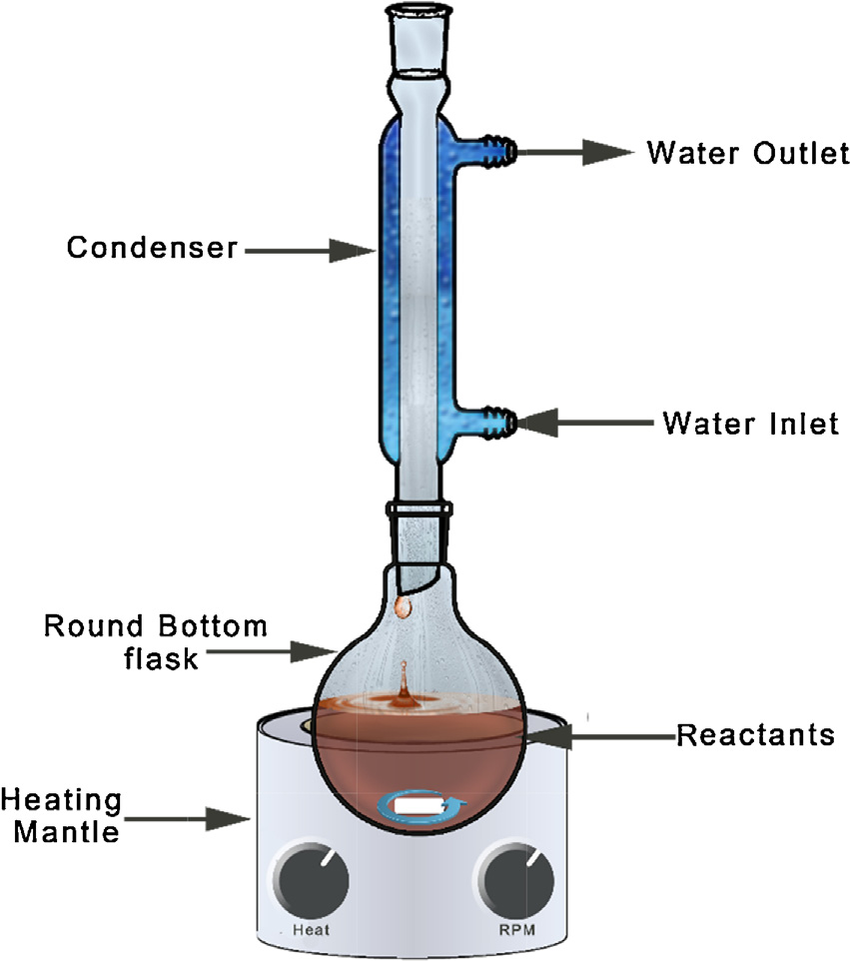
- 500 mL round bottom flask;
- Reflux condenser;
- Boiling chips;
- Retort stand and clamp for securing apparatus;
- 1 L Separatory funnel;
- Vacuum distillation setup;
- Water bath and ice;
- Heating plate;
- 100 mL x3; 250 mL x2 Beakers;
- pH indicator paper;
- Rotovap machine;
- Vacuum source;
- Laboratory scale (0.01-100 g is suitable);
- Measuring cylinder 10 mL and 100 mL.
Reagents:
- Phenylacetylmalonate (1) 48.5 mL (55.66 g, 0.20 mole) [Diethyl(phenylacetyl)malonate; cas 20320-59-6];
- Glacial acetic acid (AcOH) 60 mL;
- Concentrated sulfuric acid (H2SO4) 7.5 mL;
- Distilled water ~240 mL;
- Sodium hydroxide (NaOH) solution 20 % ~100 mL;
- Diethyl ether (Et2O) ~150 mL;
- Sodium sulfate (Na2SO4) anhydrous ~100 g;
- Drierite ~50 g (optional).
Procedure
To a diethyl phenylacetylmalonate (1) 48.5 mL (55.66 g, 0.20 mole) was added a solution of 60 mL of glacial acetic acid, 7.5 mL of concentrated sulfuric acid and 40 mL of water, and the mixture refluxed in 500 mL round bottom flask with reflux condenser for four or five hours until the decarboxylation was complete. The reaction mixture was chilled in an ice-bath, made alkaline with 20 % sodium hydroxide solution, and extracted in a separatory funnel with several portions of ether (~3 x 50 mL). The combined ethereal extracts were washed with water (~200 mL), dried with anhydrous sodium sulfate (Na2SO4) followed by Drierite (optional), and the solvent distilled off. The residue containing the ketone (2) was distilled in vacuo to give 1-Phenyl-2-Propanone (2) in 71 % yield (b.p. 97-98.5 °C/13 mmHg, 214-215 °C/760mmHg).
An Alternative Diethyl(phenylacetyl)malonate Hydrolysis to BMK and PAA (phenylacetone and phenylacetic acid)
Easy P2P synthesis from Diethyl(phenylacetyl)malonate
Diethyl(phenylacetyl)malonate Hydrolysis to BMK and PAA (phenylacetone and phenylacetic acid)...
This is an alternative synthesis method which is allow to get Phenylacetone (P2P) from Diethyl(phenylacetyl)malonate. This method consist of Diethyl(phenylacetyl)malonate alkaline hydrolysis into Phenylacetyl-malonic acid sodium salt with subsequent hydrolysis by cheap and easy available hydrochloric acid. In addition, this synthesis way takes less equipment and produce height quality product.
Equipment and glassware:
- 5L Three necked round bottom flask or batch chemical reactor;
- Top stirrer;
- Reflux condenser;
- Retort stand and clamp for securing apparatus;
- 1L Separatory funnel;
- Water bath and ice;
- 1000mL x2; 500mL x2; 250mL x2 Beakers;
- pH indicator paper;
- Laboratory scale (0.01-100 g is suitable);
- Measuring cylinder 100mL and 1000mL;
- Heating plate;
- Filter paper;
- Funnel;
- Frige;
- Glass rod;
Reagents:
- Diethyl(phenylacetyl)malonate 800g (cas 20320-59-6);
- Sodium hydroxide (NaOH) 230g;
- Distilled water 1230 ml;
- Hydrochloric acid (HCl aq);
Phenylacetyl-malonic Acid Sodium Salt From Diethyl(phenylacetyl)malonate
1. An alkali aqueous solution 50% is prepared in advance so that it has time to cool down. Sodium hydroxide 230g (NaOH) is dissolved in water 230g and cooled down to room temperature.
2. The cooled NaOH aqueous solution of is poured into a dropping funnel and this funnel is installed onto a reactor.
3. Diethyl(phenylacetyl)malonate 800g is poured into the reactor. The reaction is carried out at room temperature but a forced cooling have to be used during the hydrolysis.
4. A stirrer is turned on and cold (2-4°С) NaOH aq solution adding to the cold reaction mixture is begun slowly. The mixture is stirred for 12h. Reaction temperature has to be controlled (the mixture is self-heated). As the solution is added, reaction mixture (RM) color is changed and thickened until it is become completely thick and lumpy. The lumps is broken into a homogeneous mass.
5. RM is moved from the reactor to a vacuum filtration system funnel and filtered. Phenylacetyl-malonic acid sodium salt is obtained.
2. The cooled NaOH aqueous solution of is poured into a dropping funnel and this funnel is installed onto a reactor.
3. Diethyl(phenylacetyl)malonate 800g is poured into the reactor. The reaction is carried out at room temperature but a forced cooling have to be used during the hydrolysis.
4. A stirrer is turned on and cold (2-4°С) NaOH aq solution adding to the cold reaction mixture is begun slowly. The mixture is stirred for 12h. Reaction temperature has to be controlled (the mixture is self-heated). As the solution is added, reaction mixture (RM) color is changed and thickened until it is become completely thick and lumpy. The lumps is broken into a homogeneous mass.
5. RM is moved from the reactor to a vacuum filtration system funnel and filtered. Phenylacetyl-malonic acid sodium salt is obtained.
Phenylacetic Acid and Phenylacetone (P2P) synthesis from Phenylacetyl-malonic Acid Sodium Salt
6. Phenylacetyl-malonic acid sodium salt from the previous step is placed into the reactor and water 1L is added.
7. A reactor jacket is heated up to 60-65°С during a constant stirring. Phenylacetyl-malonic acid sodium salt has to be completely dissolved.
8. After complete dissolution, hydrochloric acid (HCl aq) is added until <3 pH. Reaction mixture is heated 60-80°С and stirred.
9. After about one hour, the phenylacetyl-malonic acid sodium salt hydrolysis is complete. The stirrer and heater are turned off. Layers are separated.
10. While the mixture is still hot, the bottom water layer is drained out and discarded. Layers can be separated by separatory funnel. The top layer is collected.
7. A reactor jacket is heated up to 60-65°С during a constant stirring. Phenylacetyl-malonic acid sodium salt has to be completely dissolved.
8. After complete dissolution, hydrochloric acid (HCl aq) is added until <3 pH. Reaction mixture is heated 60-80°С and stirred.
9. After about one hour, the phenylacetyl-malonic acid sodium salt hydrolysis is complete. The stirrer and heater are turned off. Layers are separated.
10. While the mixture is still hot, the bottom water layer is drained out and discarded. Layers can be separated by separatory funnel. The top layer is collected.
Phenylacetic Acid and Phenylacetone Separation
11. During the top layer cooling in a fridge, phenylacetic acid is crystallized. The yield is about 400g of the mixture.
12. After separation by decantation, 200g of phenylacetic acid and 200g of phenylacetone (yield is 52%) are obtained. A phenylacetone (P2P) purity is quite good for further synthesis without additional purification.
12. After separation by decantation, 200g of phenylacetic acid and 200g of phenylacetone (yield is 52%) are obtained. A phenylacetone (P2P) purity is quite good for further synthesis without additional purification.
Last edited:



