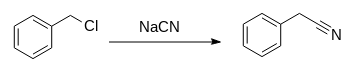
Reaction scheme:
2. The reaction mass is heated to a temperature of 85 *C, and then stirred for 6 hours.
3. Next the reactor was added purified water in an amount of 500 liters.
4. The reaction mass is heated to a temperature of 60 *C, stirred, then defended for 4 hours at the same temperature.
5. The bottom layer is transferred to the reactor, heats up to a temperature of 80 *С and trichloroethylene is distilled off (~1000 liters).
Benzyl Cyanide Synthesis From Benzyl Chloride
This video represent synthesis of benzyl cyanide from benzyl chloride with help of NaCN/KCN...
Synthesis
1. The reactor alternately load 215 liters of purified water, 126 kg of acetone, 4 kg of triethylamine, 6 kg of potassium iodide, 165 kg of sodium cyanide and 1500 litres of 30% trichloroethylene solution of benzyl chloride.2. The reaction mass is heated to a temperature of 85 *C, and then stirred for 6 hours.
3. Next the reactor was added purified water in an amount of 500 liters.
4. The reaction mass is heated to a temperature of 60 *C, stirred, then defended for 4 hours at the same temperature.
5. The bottom layer is transferred to the reactor, heats up to a temperature of 80 *С and trichloroethylene is distilled off (~1000 liters).
Last edited by a moderator:
