Phenyl-2-Propanones from Acetone Mn(III)-Catalyzed Aromatic Acetonylation
Introduction
Aside from the often amateurish reduction of (pseudo)ephedrine to methamphetamine, the most popular precursor to amphetamine and methamphetamine is phenyl-2-propanone (also called P2P, BMK, Benzyl Methyl Ketone or Phenylacetone). There is an astounding array of synthetic routes to this compound, both due to the relative simple structure of the compound, and also because of its popularity. Many of the earliest routes to the compound has been more or less abandoned due to restrictions on the pre-precursors used to make it, but there has always sprung up new methods of performing the feat of making this compound. Here is a one-step method of synthesizing phenyl-2-propanone.
- Appearance: Clear oil, flowery odor.
- Boiling Point: 214-216 °C/760mmHg, 86-87 °C/6mmHg.
- Melting Point: -15 °C.
- Molecular Weight: 134.19 g/mol.
- Density: 1.0057 g/ml (20 °C).
- Refractive Index: 1.5168.
Phenyl-2-Propanone (Phenylacetone, P2P) can be made in a single step by a free-radical reaction between benzene and acetone1,2. The reaction relies upon the special oxidative powers of manganese(III) acetate, Mn(OAc)3. Normally, when a compound is oxidized, two electrons are removed from the compound (forming a charged ion), but manganese(III) acetate is able to remove only one, creating a very reactive free radical, which reacts with almost anything nearby.
In this case, the manganese(III) acetate reacts with acetone to form an acetone radical, acetic acid and manganese(II) acetate:
In this case, the manganese(III) acetate reacts with acetone to form an acetone radical, acetic acid and manganese(II) acetate:
The formed acetone radical immediately reacts with a benzene molecule, forming a non-aromatic intermediate radical, which with the aid of a second molecule of manganese(III) acetate is oxidatively deprotonated to form phenyl-2-propanone:
As you can see, the overall reaction is that two manganese(III) acetate molecules aids the coupling of benzene and acetone, forming two acetic acid molecules in the process, while it itself is reduced to manganese(II) acetate. The manganese(II) acetate ends up in the aqueous washes of the post-reaction mixture, and the manganese(II) can be reclaimed by precipitating it as the insoluble carbonate by addition of a concentrated sodium carbonate solution, and the carbonate can be recycled into pure manganese(II)acetate again as detailed in the manganese(III) acetate.
There is also a possibility that the manganese(II) acetate can be regenerated to manganese(III) acetate in situ in the reaction mixture, either by slowly adding KMnO4 to the reaction mixture (used in the manganese(III) acetate-mediated acetonylation of cis-olefins), or by electrochemical oxidation directly in the reaction mixture (used in the manganese(III) acetate-mediated nitromethylation of benzene). However, the precise reaction conditions for these procedures in the acetonylation of benzene remains undetermined, but in the references mentioned they have found the procedures to work satisfactorily, and there seems to be no obvious reasons why it would not also work in this particular reaction. If a working manganese(III) acetate regeneration procedure is developed, this reaction should be one of the easiest, cheapest and least suspicious procedures for making phenyl-2-propanone to date.
As the formed acetone radicals are so reactive, the reaction has to be performed in a pretty dilute solution, or else the acetone radicals may combine with themselves to form dimers (with methyl ethyl ketone, almost only such dimers formed, and no addition to the benzene ring). The formed acetone radicals does not add too selectively to aromatic rings, with benzene there is only one possible product, but in the case of mono- substituted benzenes, the products were all three possible isomers, when anisole (methoxybenzene) was subjected to this treatment, the product distribution was o-methoxyphenyl-2-propanone (84.3%), m-methoxy-phenyl-2-propanone (2.6%) and p-methoxyphenyl-2-propanone (13.1%), with a 75% total yield of phenyl-2- propanones. With symmetrical disubstituted benzenes only one product is obtained, as in the preparation of 2,5-dimethoxyphenyl-2-propanone from 1,4-dimethoxybenzene (This compound is a very useful starting material for the synthesis of DOB and related psychedelic amphetamines). If alkylbenzenes such as toluene is used, benzyl acetate is produced as a by-product (in about 10-15% yield), because of the reactive nature of the benzylic carbon.
There is a possibility that the dilution of reagents used can be optimized further than the amounts described in the example below, but it is unknown how far the reduction of reagents can be reduced without side reactions occurring. In the original reference of this reaction, 0.1 mole of manganese(III) acetate dihydrate (26.8 g) was reacted with 1 mole of acetone (58 g) and 0.5 mole of benzene (39 g) in 100 ml glacial acetic acid at 70 °C to give 36% Phenyl-2-propanone, based on reacted manganese(III) acetate. As this almost five-fold increase in concentration only gave a 4% reduction in yield, there is reason to believe that the very large excesses of reactants are necessary for a successful reaction.
There is also a possibility that the manganese(II) acetate can be regenerated to manganese(III) acetate in situ in the reaction mixture, either by slowly adding KMnO4 to the reaction mixture (used in the manganese(III) acetate-mediated acetonylation of cis-olefins), or by electrochemical oxidation directly in the reaction mixture (used in the manganese(III) acetate-mediated nitromethylation of benzene). However, the precise reaction conditions for these procedures in the acetonylation of benzene remains undetermined, but in the references mentioned they have found the procedures to work satisfactorily, and there seems to be no obvious reasons why it would not also work in this particular reaction. If a working manganese(III) acetate regeneration procedure is developed, this reaction should be one of the easiest, cheapest and least suspicious procedures for making phenyl-2-propanone to date.
As the formed acetone radicals are so reactive, the reaction has to be performed in a pretty dilute solution, or else the acetone radicals may combine with themselves to form dimers (with methyl ethyl ketone, almost only such dimers formed, and no addition to the benzene ring). The formed acetone radicals does not add too selectively to aromatic rings, with benzene there is only one possible product, but in the case of mono- substituted benzenes, the products were all three possible isomers, when anisole (methoxybenzene) was subjected to this treatment, the product distribution was o-methoxyphenyl-2-propanone (84.3%), m-methoxy-phenyl-2-propanone (2.6%) and p-methoxyphenyl-2-propanone (13.1%), with a 75% total yield of phenyl-2- propanones. With symmetrical disubstituted benzenes only one product is obtained, as in the preparation of 2,5-dimethoxyphenyl-2-propanone from 1,4-dimethoxybenzene (This compound is a very useful starting material for the synthesis of DOB and related psychedelic amphetamines). If alkylbenzenes such as toluene is used, benzyl acetate is produced as a by-product (in about 10-15% yield), because of the reactive nature of the benzylic carbon.
There is a possibility that the dilution of reagents used can be optimized further than the amounts described in the example below, but it is unknown how far the reduction of reagents can be reduced without side reactions occurring. In the original reference of this reaction, 0.1 mole of manganese(III) acetate dihydrate (26.8 g) was reacted with 1 mole of acetone (58 g) and 0.5 mole of benzene (39 g) in 100 ml glacial acetic acid at 70 °C to give 36% Phenyl-2-propanone, based on reacted manganese(III) acetate. As this almost five-fold increase in concentration only gave a 4% reduction in yield, there is reason to believe that the very large excesses of reactants are necessary for a successful reaction.
Experiment
A mixture of Manganese(III) acetate dihydrate (13.4 g, 50 mmol), benzene (150 ml), acetone (150 ml) and glacial acetic acid (250 ml) was refluxed under an inert atmosphere (nitrogen, argon or helium) until the dark brown color of manganese(III) acetate changed to the pale pink of manganese(II) acetate (about 90 min). You may try to carry out reaction under air atmosphere, but be prepared to get less yield. The reaction mixture was partitioned between 400 ml ether and 250 ml water. The ether layer was separated and washed with 250 ml water and with 2x250 ml 5% Na2CO3 to remove any remaining acetic acid. The ether was then dried over anhydrous Na2SO4 (or MgSO4), the solvent evaporated and the residue fractionally distilled to recover unreacted benzene, and to give phenyl-2-propanone in 40% yield (1.34 g) based on the reacted manganese(III) acetate, which is the limiting reagent in this reaction.
If anhydrous Manganese(III) acetate is used, 50 mmol corresponds to 10.1 g instead of the 13.4 g of the dihydrate. When solid aromatics was used as substrates, the amounts used were reduced to facilitate the work-up process. In the case of 1,4-dimethoxybenzene, 100 g (720 mmol) was used instead. Of course, any unreacted starting material is recovered by distillation after the reaction and reused in another run.
The variations in reaction time for different substrates, as well as the yields (with product distribution in the case of monosubstituted aromatics) are presented in the table below.
If anhydrous Manganese(III) acetate is used, 50 mmol corresponds to 10.1 g instead of the 13.4 g of the dihydrate. When solid aromatics was used as substrates, the amounts used were reduced to facilitate the work-up process. In the case of 1,4-dimethoxybenzene, 100 g (720 mmol) was used instead. Of course, any unreacted starting material is recovered by distillation after the reaction and reused in another run.
The variations in reaction time for different substrates, as well as the yields (with product distribution in the case of monosubstituted aromatics) are presented in the table below.
Preparation of Manganese(III)acetate
Manganese(III) acetate from KMnO4Manganese(III) acetate can be made from any water-soluble manganese(II) salt, or from the corresponding acid soluble hydroxide (Mn(OH)2) and oxide (MnO) by precipitating it as the carbonate salt, and then boiling it in acetic acid to form manganese(II)acetate, which is finally oxidized to manganese(III)acetate by potassium permanganate. Potassium permanganate can also be used as a starting material itself, by reducing it with hexamine or formaldehyde solution. All manganese(II) salts are susceptible to oxidation to manganese(IV) by oxygen, so try to exclude air as conveniently possible from these compounds and their solutions.
Theory of the reaction
At first, the Hexamine is hydrolyzed by HCl to ammonium chloride and formaldehyde:
C6H12N4 + 4 HCl → 6 HCHO + 4 NH4Cl
The KMnO4 is then reduced to Mn2+ in acidic solution, when the formaldehyde is oxidized to the acid:
2 KMnO4 + 5 HCHO + 6 HCl → 2 MnCl2 + 2 KCl + 5 HCOOH + 3 H2O
Or alternatively, by the formaldehyde being oxidized all the way to carbon dioxide:
4 KMnO4 + 5 HCHO + 12 HCl → 4 MnCl2 + 4 KCl + 5 CO2 + 16 H2O
The MnCl2 in the solution is then precipitated as the water-insoluble Manganese(II)carbonate:
MnCl2 + 2 NaHCO3 → 2 NaCl + MnCO3 + CO2 or MnCl2 + Na2CO3 → 2 NaCl + MnCO3
The carbonate is then converted to the Manganese(II)acetate
MnCO3 + 2 AcOH → Mn(OAc)2 + CO2 + H2O
As the last step, potassium permanganate oxidizes Manganese(II)acetate to Manganese(II)acetate
4 Mn(OAc)2 + 5 HOAc + KMnO4 → 4 Mn(OAc)3 + KOAc + MnO2
Thus, it can be concluded that either hexamine or formaldehyde can be used for the reduction, and that either sodium bicarbonate (baking soda, NaHCO3) or sodium carbonate (washing soda, Na2CO3), if you balance the proportions of the reactants appropriately.
Theory of the reaction
At first, the Hexamine is hydrolyzed by HCl to ammonium chloride and formaldehyde:
C6H12N4 + 4 HCl → 6 HCHO + 4 NH4Cl
The KMnO4 is then reduced to Mn2+ in acidic solution, when the formaldehyde is oxidized to the acid:
2 KMnO4 + 5 HCHO + 6 HCl → 2 MnCl2 + 2 KCl + 5 HCOOH + 3 H2O
Or alternatively, by the formaldehyde being oxidized all the way to carbon dioxide:
4 KMnO4 + 5 HCHO + 12 HCl → 4 MnCl2 + 4 KCl + 5 CO2 + 16 H2O
The MnCl2 in the solution is then precipitated as the water-insoluble Manganese(II)carbonate:
MnCl2 + 2 NaHCO3 → 2 NaCl + MnCO3 + CO2 or MnCl2 + Na2CO3 → 2 NaCl + MnCO3
The carbonate is then converted to the Manganese(II)acetate
MnCO3 + 2 AcOH → Mn(OAc)2 + CO2 + H2O
As the last step, potassium permanganate oxidizes Manganese(II)acetate to Manganese(II)acetate
4 Mn(OAc)2 + 5 HOAc + KMnO4 → 4 Mn(OAc)3 + KOAc + MnO2
Thus, it can be concluded that either hexamine or formaldehyde can be used for the reduction, and that either sodium bicarbonate (baking soda, NaHCO3) or sodium carbonate (washing soda, Na2CO3), if you balance the proportions of the reactants appropriately.
Experiment
15 g KMnO4 was dissolved in some hot water and combined w/a soln of 15 g hexamine. Nothing happened. Then conc. HCl was added in portions w/swirling until mixtr acidic. As he did it, the mixtr gradually turned completely clear and the stench of formaldehyde became almost unbearable. Then you have to put the soln in a 5 liter jar and mixed it w/a molar excess of saturated NaHCO3 (you can calculate it if you want - I just took a large xcess). White precipitate (MnCO3) forms. The jar was filled w/water to wash it. DO NOT use NaOH for precip'ing manganese - Mn(OH)2 instantly oxidizes when exposed to air, and the carbonate is pretty stable.
When all settled, the water was decanted into another jar (to collect the still remaining suspended MnCO3 later) and the precip't washed in the same manner once again, MnCO3 in the other jar washed later too. Then all combined, water decanted off, precip't vac. filtered - do not dry it - there's no need for that, and it will oxidize somewhat during drying. Just scrape it off the filter and dump into some GAA. Now, guess what, there was no fizzling and bubbling at this stage, in fact, to dissolve all the MnCO3 it took a 2-hour reflux. At this stage, the solution was a pleasant pink color, just as it should have been.
The GAA (Glacial Acetic Acid) was boiled off overnight at 130 °C in an oil bath. I found that the bottom of the flask was covered w/some white (not pink) crystals in the next morning - well, I thought, that must be unhydrous Mn(OAc)2 (the pink stuff is a tetrahydrate) - and so it turned out to be. I don't remember the exact weight (21 g?) but the yield was quantitative.
Now I went on to dissolving his salt in GAA, 250 ml (I thought you'd have to 1st evap. GAA and then dissolve in it again as the former one has water in it - but he was wrong, as you'll see. Well, he needed to weigh it anyway. Just skip this evap'n/dissolution step, it's probably not needed) - It doesn't dissolve! even w/reflux! Probably, the unhydrous stuff doesn't, so he added a theoretical amount of water, well, a little more in fact, about 7 ml to the mxtr and w/some boiling most of it went in the soln, some was still left at the bottom - it was OK, as it turned.
To this boiling solution, I added about 5,4 g (~1/4 molar equivalent) of KMnO4, in, say, 6 portions. During and after each addition, the mixture was vigorously stirred with a glass rod. The KMnO4 must be prior to that ground as fine as possible - it's not hard at all. The mixture turned dark and opaque. Boiling was continued for some 10 min, then the flask put into the fridge (16 °C) and 3 ml water added to it to induce crystallization. The walls of the flask were periodically furiously scratched (on the inside) with a glass rod and after like 10 hours a heavy crop of dark brown, as they should be, crystals precipitated. (If it doesn't, you can add 3 ml more water to it and w/some scratching it'll happen in an hour).
The mixture was filtered, the mother liquor still very dark (put 3 ml water into it and let stand for a week - all of your product will crystallize, and the liquor will turn colorless, this time the crystals will be not dark brown, but of rust/cinnamon powder color, just like the dry stuff), crystals sucked as dry as possible and dried for ~3 days in a dessiccator over CaO. Dry Mn(AcO)3 is a very, very fine rust-colored powder, it's unbelievable how much it increases in volume comparing to the initial KMnO4! Still, the weight is consistent - I got about 22 g from the 1st crop, and the 2nd one is still in the flask, probably will be ~5-7 g - the original article states the yield of 85%, fits w/my result perfectly.
When all settled, the water was decanted into another jar (to collect the still remaining suspended MnCO3 later) and the precip't washed in the same manner once again, MnCO3 in the other jar washed later too. Then all combined, water decanted off, precip't vac. filtered - do not dry it - there's no need for that, and it will oxidize somewhat during drying. Just scrape it off the filter and dump into some GAA. Now, guess what, there was no fizzling and bubbling at this stage, in fact, to dissolve all the MnCO3 it took a 2-hour reflux. At this stage, the solution was a pleasant pink color, just as it should have been.
The GAA (Glacial Acetic Acid) was boiled off overnight at 130 °C in an oil bath. I found that the bottom of the flask was covered w/some white (not pink) crystals in the next morning - well, I thought, that must be unhydrous Mn(OAc)2 (the pink stuff is a tetrahydrate) - and so it turned out to be. I don't remember the exact weight (21 g?) but the yield was quantitative.
Now I went on to dissolving his salt in GAA, 250 ml (I thought you'd have to 1st evap. GAA and then dissolve in it again as the former one has water in it - but he was wrong, as you'll see. Well, he needed to weigh it anyway. Just skip this evap'n/dissolution step, it's probably not needed) - It doesn't dissolve! even w/reflux! Probably, the unhydrous stuff doesn't, so he added a theoretical amount of water, well, a little more in fact, about 7 ml to the mxtr and w/some boiling most of it went in the soln, some was still left at the bottom - it was OK, as it turned.
To this boiling solution, I added about 5,4 g (~1/4 molar equivalent) of KMnO4, in, say, 6 portions. During and after each addition, the mixture was vigorously stirred with a glass rod. The KMnO4 must be prior to that ground as fine as possible - it's not hard at all. The mixture turned dark and opaque. Boiling was continued for some 10 min, then the flask put into the fridge (16 °C) and 3 ml water added to it to induce crystallization. The walls of the flask were periodically furiously scratched (on the inside) with a glass rod and after like 10 hours a heavy crop of dark brown, as they should be, crystals precipitated. (If it doesn't, you can add 3 ml more water to it and w/some scratching it'll happen in an hour).
The mixture was filtered, the mother liquor still very dark (put 3 ml water into it and let stand for a week - all of your product will crystallize, and the liquor will turn colorless, this time the crystals will be not dark brown, but of rust/cinnamon powder color, just like the dry stuff), crystals sucked as dry as possible and dried for ~3 days in a dessiccator over CaO. Dry Mn(AcO)3 is a very, very fine rust-colored powder, it's unbelievable how much it increases in volume comparing to the initial KMnO4! Still, the weight is consistent - I got about 22 g from the 1st crop, and the 2nd one is still in the flask, probably will be ~5-7 g - the original article states the yield of 85%, fits w/my result perfectly.
Phenyl-2-Propanone from Chloroacetone
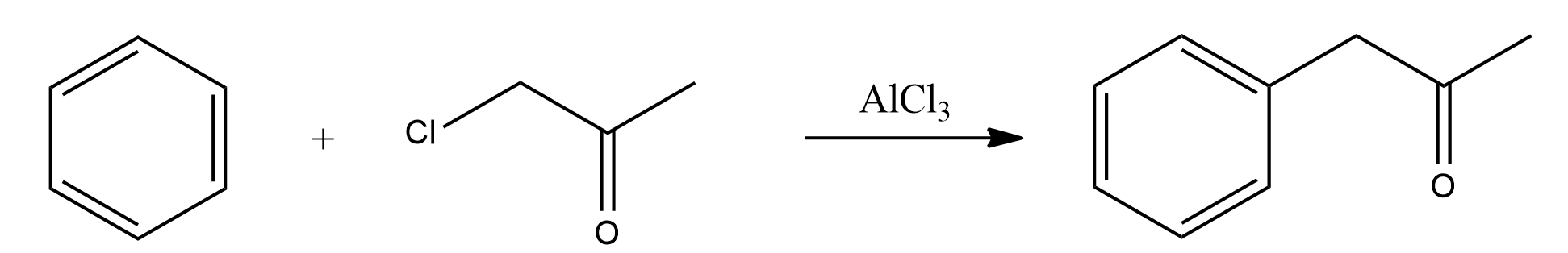
41 grams (0.31 mole) of anhydrous aluminum chloride and 100 ml of anhydrous benzene (free from thiophene) were put in a 500 ml three-necked flask which was equipped with a mercury-sealed stirrer, a reflux water condenser and a small addition funnel. The top of the condenser was connected to a sulfuric acid trap, and this trap was connected to a gas absorption bottle. The mixture was stirred and heated to refluxing on a steam bath, and 13.9 g (0.15 mole) of chloroacetone was allowed to drop in slowly during a period of 30 minutes. After refluxing for 5 hours, the solution was practically black. After cooling to room temperature, the reaction mixture was decomposed by slowly adding water through the condenser, stirring during the addition. When no more hydrogen chloride was evolved, 20 ml of water and 20 ml of concentrated hydrochloric acid was added. The benzene layer was separated, and the aqueous layer extracted with four 25 ml portions of benzene. All the benzene solutions were combined and filtered. The benzene was distilled off, and the remaining viscous oil was distilled under reduced pressure. Nine grams of liquid boiling below 123 °C/20-22 mmHg was obtained. Approximately 10 g of high-boiling material was left in the distilling flask. Phenyl-2-Propanone was recovered from the distillate by making the bisulfite addition product, filtering, decomposing the addition product with sodium carbonate solution, and steam distilled as long as any oil distilled over. The distillate was extracted with ether, the ether dried over anhydrous MgSO4 and the ether distilled on a steam bath. The phenyl-2-Propanone was distilled under reduced pressure, bp 108-114 °C/20-22mmHg. Yield 6.5 g (32%).
There is laboratory method by producing P2P from acetone and benzene via bromine acetone.
Preparation of Chloroacetone and Bromoacetone
ChloroacetoneGood method of preparation 150 ml acetone 50 ml water 12 g Cupric chloride 6 g lithium chloride. Reflux till reaction completes. Literature states 24 hours, but the reaction has a half-life of about 24 minutes at 20 °C (same article half of marker in 24 minutes, the marker being oxygen consumption in a slightly different reaction). Therefore, 5 hours is probably sufficient for reflux.
After reacting, distill everything below 123 °C. The still bottoms can be reprocessed to recover cuprous chloride and lithium chloride. Both can be recovered by dissolving with minimum water. The mix is easily converted to cupric chloride-lithium chloride by boiling with 20-35% hydrochloric acid.
Redistill slowly through a packed column to remove acetone. This leaves two fractions one distilling at 89 °C. which is water and chloroacetone and the second distilling at 121 °C which is ?pure? chloroacetone. The second fraction may contain unsymmetrical dichloroacetone I haven't had a sample analyzed. Calcium Chloride will crash the water-chloroacetone mix, which tends to form a colloidal solution.
Chloroacetone must be stabilized with 1% calcium carbonate or 0.1% water if it is stored, or it forms an explosive sludge. Distillation of a water-chloroacetone mix at 89 °C is the most efficient way of separating unsym-dichloroacetone from commercial products.
Chloroacetone
This produces a product absolutely free from polychlorinated acetone, which usually is formed in the chlorination of acetone, and is almost impossible to completely remove by distillation.
A dried ether solution (approximately 500 ml) containing 0.5 mole of diazomethane was placed in a 1000 ml three-necked flask and practical grade acetyl chloride (0.25 mole) was added slowly from a dropping funnel with constant stirring of the solution which was maintained at a temperature not greater than 5 °C. The reaction mixture was allowed to stand for two hours after the addition of the acetyl chloride and was then saturated with anhydrous HCl over a period of two hours. The bulk of the ether was removed by distillation, and the residual solution fractionated through a small column. The product boiling at 118-119 °C at weighed 15.8 g (68%), d 1.126.
Bromoacetone
A 5 L, three-necked, round-bottomed flask is provided with an efficient mechanical stirrer, a 48 cm. Allihn reflux condenser, a thermometer, and a 500 ml separatory funnel, the stem of which reaches nearly to the bottom of the flask.
Through the separatory funnel are introduced 1.6 L of water, 500 ml of pure acetone, and 372 ml of glacial acetic acid. The stirrer is started and the temperature of the water bath is raised to 70-80 °C, so that the mixture in the flask is at about 65 °C. Then 354 ml (7.3 moles) of bromine is carefully added through the separatory funnel. The addition, which requires one to two hours, is so regulated as to prevent the accumulation of unreacted bromine. As a rule, the solution is decolorized in about twenty minutes after the bromine has been added. When the solution is decolorized, it is diluted with 800 ml of cold water, cooled to 10 °C, made neutral to Congo red with about 1 kg. of solid anhydrous sodium carbonate, and the oil which separates are collected in a separatory funnel and dried with 80 g of anhydrous calcium chloride. After drying, the oil is fractionated and the fraction boiling at 38-48 °C/13 mmHg is collected. The yield is 470-480 g. (50-51% yield). If a purer product is desired, the above product is refractionated and the fraction boiling at 40-42 °C/13 mmHg is collected. The yield is 400-410 g. (43-44% yield).
The higher-boiling fraction contains a mixture of isomeric dibromoacetones.
After reacting, distill everything below 123 °C. The still bottoms can be reprocessed to recover cuprous chloride and lithium chloride. Both can be recovered by dissolving with minimum water. The mix is easily converted to cupric chloride-lithium chloride by boiling with 20-35% hydrochloric acid.
Redistill slowly through a packed column to remove acetone. This leaves two fractions one distilling at 89 °C. which is water and chloroacetone and the second distilling at 121 °C which is ?pure? chloroacetone. The second fraction may contain unsymmetrical dichloroacetone I haven't had a sample analyzed. Calcium Chloride will crash the water-chloroacetone mix, which tends to form a colloidal solution.
Chloroacetone must be stabilized with 1% calcium carbonate or 0.1% water if it is stored, or it forms an explosive sludge. Distillation of a water-chloroacetone mix at 89 °C is the most efficient way of separating unsym-dichloroacetone from commercial products.
Chloroacetone
This produces a product absolutely free from polychlorinated acetone, which usually is formed in the chlorination of acetone, and is almost impossible to completely remove by distillation.
A dried ether solution (approximately 500 ml) containing 0.5 mole of diazomethane was placed in a 1000 ml three-necked flask and practical grade acetyl chloride (0.25 mole) was added slowly from a dropping funnel with constant stirring of the solution which was maintained at a temperature not greater than 5 °C. The reaction mixture was allowed to stand for two hours after the addition of the acetyl chloride and was then saturated with anhydrous HCl over a period of two hours. The bulk of the ether was removed by distillation, and the residual solution fractionated through a small column. The product boiling at 118-119 °C at weighed 15.8 g (68%), d 1.126.
Bromoacetone
A 5 L, three-necked, round-bottomed flask is provided with an efficient mechanical stirrer, a 48 cm. Allihn reflux condenser, a thermometer, and a 500 ml separatory funnel, the stem of which reaches nearly to the bottom of the flask.
Through the separatory funnel are introduced 1.6 L of water, 500 ml of pure acetone, and 372 ml of glacial acetic acid. The stirrer is started and the temperature of the water bath is raised to 70-80 °C, so that the mixture in the flask is at about 65 °C. Then 354 ml (7.3 moles) of bromine is carefully added through the separatory funnel. The addition, which requires one to two hours, is so regulated as to prevent the accumulation of unreacted bromine. As a rule, the solution is decolorized in about twenty minutes after the bromine has been added. When the solution is decolorized, it is diluted with 800 ml of cold water, cooled to 10 °C, made neutral to Congo red with about 1 kg. of solid anhydrous sodium carbonate, and the oil which separates are collected in a separatory funnel and dried with 80 g of anhydrous calcium chloride. After drying, the oil is fractionated and the fraction boiling at 38-48 °C/13 mmHg is collected. The yield is 470-480 g. (50-51% yield). If a purer product is desired, the above product is refractionated and the fraction boiling at 40-42 °C/13 mmHg is collected. The yield is 400-410 g. (43-44% yield).
The higher-boiling fraction contains a mixture of isomeric dibromoacetones.
Last edited:



