G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 3,058
- Solutions
- 3
- Reaction score
- 3,492
- Points
- 113
- Deals
- 1
Introduction
How to turn grams into milliliters and vice versa?
What is moles?
What is molar mass?
How to turn moles into grams and vice versa?
Another example, you have to add 10 moles of sodium borohydride (NaBH4) with 37.83 g/moles molecular mass to the reaction and you need to count it to gramms:
How to count reagents?
How to count a reaction yield?
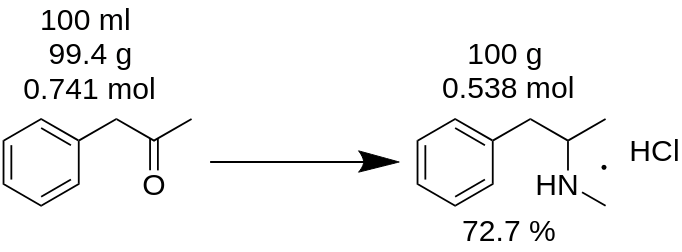
Then, count how much moles of a lack reagent (P2P in this example) take part in the reaction.
I get a lot of same questions about reactions yields, amount of reagents, how to turn one dimension to another one and notice that people don't know how to count these simple but very important numbers correctly. I decided to describe correct methods in this topic. If you don't understand something, don't hesitate to ask and discuss your questions in comment section.
How to turn grams into milliliters and vice versa?
The gram (SI unit symbol g) is a mass unit in the International System of Units (SI) equal to one one thousandth of a kilogram (1/1000). Gram is the absolute weight of a volume of pure water equal to the cube of the hundredth part of a metre [1 cm3], and at the temperature of melting ice, the defining temperature (~0 °C) was later changed to 4 °C, the temperature of maximum density of water.
Conversion factors
Conversion factors
- 1 gram (g) = 15.4323583529 grains (gr);
- 1 grain (gr) = 0.06479891 grams;
- 1 avoirdupois ounce (oz) = 28.349523125 grams;
- 1 troy ounce (ozt) = 31.1034768 grams;
- 100 grams (g) = 3.527396195 ounces (oz);
- 1 carat (ct) = 0.2 grams;
- 1 gamma (γ) = 10−6 grams;
- 1 undecimogramme = 1 "eleventh-gram" = 10−11 grams in the historic quadrant–eleventh-gram–second system (QES system) a.k.a. hebdometre–undecimogramme–second system (HUS system);
- 500 grams (g) = 1 jin in the Chinese units of measurement;
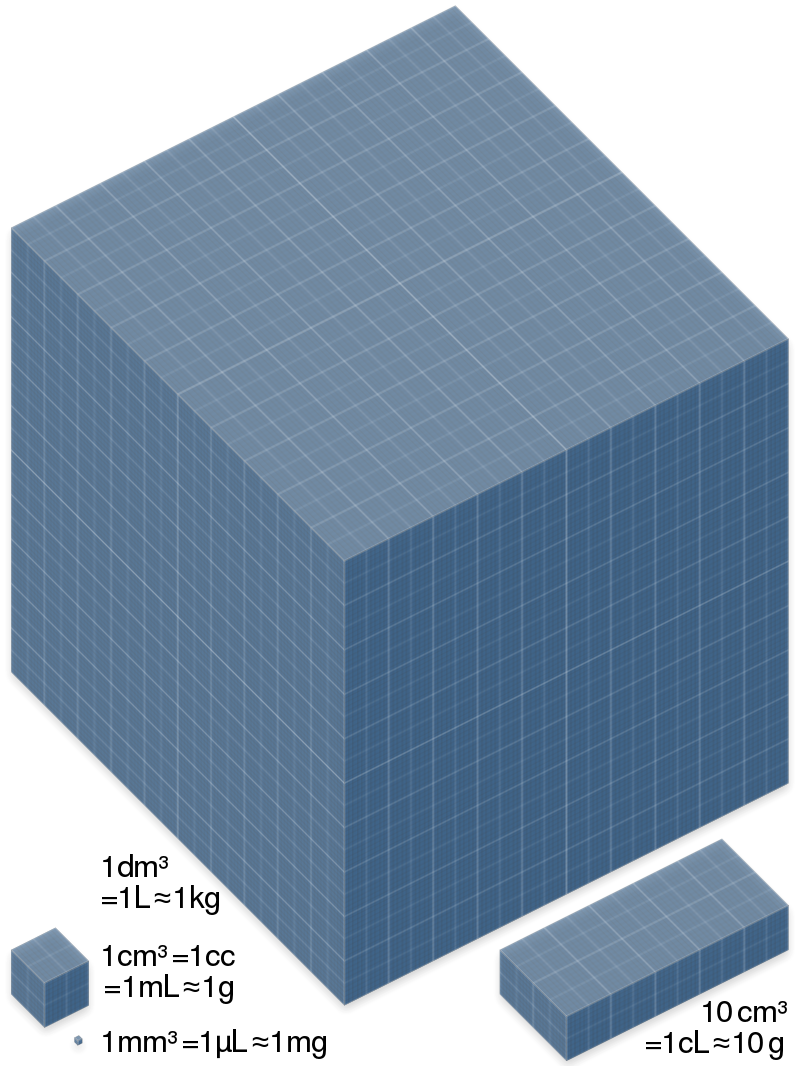
The litre (international spelling) or liter (American English spelling), SI symbols L and l. Liter is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metre (m3). A cubic decimetre (or litre) occupies a volume of 10 cm × 10 cm × 10 cm (see figure) and is thus equal to one-thousandth of a cubic metre. Milliliter, SI symbol ml or mL is cubic centimetre. A cubic centimeter (or ml) occupies a volume of 1 cm × 1 cm × 1 cm and is thus equal to one one thousandth of a liter (1/1000).
Conversion factors
Conversion factors
- 1 Imperial quart = 1.1365225 L;
- 1 U.S. quart = 0.946352946 L;
- 1 Imperial pint = 0.56826125 L;
- 1 U.S. pint = 0.473176473 L;
- 1 Imperial gallon = 4.54609 L;
- 1 U.S. gallon = 3.785411784 L;
- 1 cubic foot = 28.316846592 L;
- 1 cubic inch = 0.016387064 L;
- 1 Imperial fluid ounce = 28.4130625 mL;
- 1 U.S. fluid ounce = 29.5735295625 mL;
Grams can be easily turned into milliliters according this formula:
V = m / ρ,
where m — mass of the substance, g; ρ — density of the substance, g/ml; V — volume of the substance.
According with this formula, you can turn every substance into volume and vice versa. For instance, you have 20 g of mercury (Hg) and you want to calculate its mass. ρ (Density) of mercury is 13.5 g/ml, hence
According with this formula, you can turn every substance into volume and vice versa. For instance, you have 20 g of mercury (Hg) and you want to calculate its mass. ρ (Density) of mercury is 13.5 g/ml, hence
V(Hg) = 20 g / 13.5 g/ml = 1.48 ml
1.48 ml Is the volume of 20 g of mercury.
What is moles?
The mole, symbol mol, n, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample (in any substance). The mole is defined as containing exactly 6.02214085774 × 10²³ particles (atoms, molecules, ions, electrons or any other objects). For example, 10 moles of water (a chemical compound H2O) and 10 moles of mercury (a chemical element Hg), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. Anyway, that's quite a lot, isn't it? This is the reason why it's more convenient to know how to convert grams to moles instead of grams to the number of atoms.
What is molar mass?
Molar mass is a characteristic of a substance, the ratio of the mass of a substance to its quantity. Numerically equal to the mass of 1 mole of a substance, that is, the mass of a substance contains a number of particles equal to Avogadro's number. The molar mass, expressed in g/mol, numerically coincides with the molecular mass, expressed in r.a.m., and relative atomic mass. However, there is difference between molar mass and molecular mass, they are numerically equal only and differ in dimension.
For example, the molar mass of oxygen as an element M(O) = 16 g/mol, but as a simple substance consisting of molecules O2 = 32 g/mol.
The molar masses of complex molecules can be determined by summing up the molar masses of their constituent elements. For example, the molecular mass of water H2O is:
For example, the molar mass of oxygen as an element M(O) = 16 g/mol, but as a simple substance consisting of molecules O2 = 32 g/mol.
The molar masses of complex molecules can be determined by summing up the molar masses of their constituent elements. For example, the molecular mass of water H2O is:
M(H2O) = 2 x M(H) + M(O) = 2 x 1 g/mol + 16 g/mol = 18 g/mol.
How to turn moles into grams and vice versa?
To correctly evaluate the number of moles, n, of a substance of a specific mass, m, (in grams), you need to follow the grams to moles formula:
n = m / M,
where: M - the molar mass of this material. The unit is typically g/mol; m - mass of the substance, g; n - moles of the substance, mol.
For instace, you have 100 g of P2NP, which has 163.17 g/mol molecular mass. You need to cound how much moles it is. According to formula above:
For instace, you have 100 g of P2NP, which has 163.17 g/mol molecular mass. You need to cound how much moles it is. According to formula above:
n(P2NP) = 100 g / 163.17 g/mol = 0.6129 moles.
Another example, you have to add 10 moles of sodium borohydride (NaBH4) with 37.83 g/moles molecular mass to the reaction and you need to count it to gramms:
m(NaBH4) = 10 moles x 37.83 g/mol = 378.3 g.
How to count reagents?
You have opened synthesis manual and you realized that you need much less or bigger scale synthesis. You a confused by numbers and don't know how to count them to your scale? There is an explanation.
In case you want to increase a synthesis scale and you sure that it is possible to do according to linear dependence, you just have to multiply all reagents amounts by the same index. You'll get amounts of reagents for your synthesis scale and you don't need to ask any Expert about this!
Example: you want to carry out Amphetamine synthesis via NaBH4/CuCl2 and you have to take 1000 g of P2NP for this synthesis according with manual from BB forum. You want to carry out 150 g P2NP synthesis load. Your algorithm is:
In case you want to increase a synthesis scale and you sure that it is possible to do according to linear dependence, you just have to multiply all reagents amounts by the same index. You'll get amounts of reagents for your synthesis scale and you don't need to ask any Expert about this!
Example: you want to carry out Amphetamine synthesis via NaBH4/CuCl2 and you have to take 1000 g of P2NP for this synthesis according with manual from BB forum. You want to carry out 150 g P2NP synthesis load. Your algorithm is:
1. Divide 1000 g of described in the manual amount of main precursor P2NP by 150 g and you'll get necessary index 6.67.
2. Divide all reagent amounts by 6.67 and you'll reach your goal.
In case you need to scale up this synthesis load from 1000 g P2NP to 2500 g, follow these instructions:
1. Divide 2500 g P2NP by 1000 g and you'll get index 2.5.
2. Multiply all reagents by 2.5 and you'll reach your goal.
How to count a reaction yield?
You have carried out methamphetamine hydrochloride synthesis from 100 ml P2P and got 100 g of the product. You think that you get 100% yield? It is wrong!
Example:
Example:
First of all, you have to count mass of P2P, which was used for this synthesis.
m(P2P) = 100 ml / 1.006 = 99.4g
Then, count how much moles of a lack reagent (P2P in this example) take part in the reaction.
n(P2P) = 99.4 g / 134.178 g/mole = 0.741 moles.
A lack reagent is reagent, which takes part in a reaction and the one with a smallest moles amount. For example, in reaction of P2P reductive amination to methamphetamine, you have to take 1 mole of P2P and ~3.5 moles of methylamine. P2P is lack reagent in this reaction.
According with the reaction balance, 1 mole of P2P gives 1 mole of methamphetamine hydrochloride. Hence, 0.741 moles of P2P gives 0.741 moles of methamphetamine hydrochloride (MH), which is
According with the reaction balance, 1 mole of P2P gives 1 mole of methamphetamine hydrochloride. Hence, 0.741 moles of P2P gives 0.741 moles of methamphetamine hydrochloride (MH), which is
m(MH) = 0.741 moles x 185.69 g/mole = 137.56 g,
where 185.69 g/mole is molecular mass of methamphetamine hydrochloride.
In known as theoretical yield. Theoretical yield is amount of substance, which have to be obtained as the reaction result. Therefore, you can count reaction yield from this theoretical result:
In known as theoretical yield. Theoretical yield is amount of substance, which have to be obtained as the reaction result. Therefore, you can count reaction yield from this theoretical result:
Yield, % = (m(theory)*100)/m(practice),
Yield (MH) = (100 g * 100) / 137.56 = 72.7 %.
Yield (MH) = (100 g * 100) / 137.56 = 72.7 %.
As you can see, real yield 72.7 % is really different from 100 % yield, which was mistakenly calculated from reaction mass. It pays significant role in any substance production.
Last edited:
