G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,693
- Solutions
- 3
- Reaction score
- 2,818
- Points
- 113
- Deals
- 1
Introduction
I would like to present two methods for producing nitromethane using inexpensive equipment and utensils from available reagents.
The most famous and widely used method for producing nitromethane in practice is the interaction of chloroacetic acid and sodium nitrite in an aqueous solution and at high temperatures, called the Kolbe method. The process is carried out as follows. An alkaline agent (sodium carbonate, potassium carbonate, ammonia, etc.) is added to an aqueous solution of chloroacetic acid until the chloroacetic acid is completely neutralized. An aqueous solution of sodium nitrite is added to the reaction mass, and the mixture of components is gradually heated until the start of distillation of nitromethane. At 80-85 °C, as soon as carbon dioxide bubbles begin to evolve, the heating is stopped. At this temperature, the reaction continues without external heating, and the formed nitromethane is distilled off with water in the form of heavy oily droplets. As the distillation proceeds, the temperature rises to 100 °C. The alkaline environment that occurs during the reaction leads to a decrease in the yield of nitromethane, which is a disadvantage of the above method. The nitromethane yield is no more than 40% of theory.
The most famous and widely used method for producing nitromethane in practice is the interaction of chloroacetic acid and sodium nitrite in an aqueous solution and at high temperatures, called the Kolbe method. The process is carried out as follows. An alkaline agent (sodium carbonate, potassium carbonate, ammonia, etc.) is added to an aqueous solution of chloroacetic acid until the chloroacetic acid is completely neutralized. An aqueous solution of sodium nitrite is added to the reaction mass, and the mixture of components is gradually heated until the start of distillation of nitromethane. At 80-85 °C, as soon as carbon dioxide bubbles begin to evolve, the heating is stopped. At this temperature, the reaction continues without external heating, and the formed nitromethane is distilled off with water in the form of heavy oily droplets. As the distillation proceeds, the temperature rises to 100 °C. The alkaline environment that occurs during the reaction leads to a decrease in the yield of nitromethane, which is a disadvantage of the above method. The nitromethane yield is no more than 40% of theory.
Pure Nitroethane Liquid
One of the promising methods for producing nitromethane is a method based on the reaction of dimethyl sulfate with sodium nitrite at elevated temperatures in a water solvent. The method is characterized by the presence of easy accessibility material base and high quality of the obtained nitromethane, which makes it possible to use it in clandestine laboratories for large loads. The essence of the method according to the above method consists in heating a mixture of starting components in the water and an alkaline agent in two stages: the first stage of the process (the formation of approximately three-fourths the amount of nitromethane and sodium monomethyl sulfate) is carried out at temperatures up to 85-100 °C with further distillation of the resulting nitromethane. The second stage of the process (reaction between sodium monomethyl sulfate and sodium nitrite with the formation of nitromethane) is carried out at a temperature of 110 - 120 °C, and sometimes up to 160-200 °C with constant distillation of nitromethane.
Appearance: colorless, oily liquid; light, fruity odor
Melting Point: -28.7 °C
Molecular Weight: 61.042 g/mol
Density: 1.1371 g/ml (20 °C)
Refractive Index: 1.3817 at 20 °C/D
Safety note: Hold all manipulations in pull out probe or under exhaust hood; Nitromethane is a toxic substance. When vapors are inhaled in high concentrations, nitromethane damages the liver and kidneys, and has a harmful effect on the central nervous system. A drug that also has a convulsive effect and aftereffects. LD50 = 950 mg / kg for mice. Dimethyl sulfate is a highly toxic substance with skin-resorptive effects, LD50 140 mg / kg (mice, oral). Vapors of dimethyl sulfate have a strong irritating and cauterizing effect on the mucous membranes of the respiratory tract and eyes (inflammation, edema, respiratory tract damage, conjunctivitis). It also has a general toxic effect, especially on the central nervous system. Liquid dimethyl sulphate, when in contact with the skin, causes burns and long-lasting ulcers, in high concentrations, necrosis (necrosis). Toxicity is due to hydrolysis products, which include methanol and sulfuric acid. Like other chloroacetic acids and related halocarbons, chloroacetic acid is a hazardous alkylating agent. The LD50 for rats is 76 mg/kg. Using chemical glass, gloves, chemical coat and respiratory mask is required.
Equipment and glassware:
- Two- or three-necked flask 500 ml;
- Beakers 100 ml (x2) and 200 ml (x2);
- Ice bath (0 °C);
- Magnetic stirrer with heater or Top mixer with heating plate (in a pair of three-necked flask and Bunsen burner);
- Laboratory grade thermometer (0 °C to 200 °C) with flask adapter;
- Small conventional funnel (d 10 cm);
- Laboratory scale (0.1 g-100 g is suitable);
- Filter paper;
- Measuring cylinder for 100 ml;
- Boiling stones;
- Liebig condenser;
- Retort stand and clamp for securing apparatus;
- Connection tube;
- Distillation adapter;
- Receiver flask (Erlenmeyer flask 100 ml with cap);
- Separator funnel 500 ml;
- Erlenmeyer flask 100 ml x2 and 200 ml x2.
Nitromethane synthesis from MCA (monochloroacetic acid)
In a two-necked flask 500 ml, place 97.2 g (1.05 mol) of monochloroacetic acid, dissolve it in 200 ml of distilled water and gradually add 60 g of anhydrous sodium carbonate (in portions of 1 g), gently stirring solution. The rapid addition of large amounts of sodium carbonate leads to lumps formation. Separately dissolve, slightly warming up, 71.1 g (1.03 mol) of sodium nitrite in 120 ml of water, cool the solution in an ice bath and add it with stirring to the solution of sodium chloroacetate. Then boiling stones are introduced into the flask, the thermometer is immersed in liquid. The thermometer dipping in the liquid is absolutely necessary, as the success of the preparation depends on proper temperature control. The vital point of the whole preparation is to remove all external heat as soon as the reaction is well started. This is between 80 and 85 °C. No frothing ever occurs when this precaution is rigidly adhered to. The receiver is cooled in ice water bath (Fig. 1). The flask is placed on a grid and gently heated on an electric stove or with a Bunsen burner through a wire grid. The liquid in the flask turns yellow, then turns green, and finally becomes brown in color. When solution temperature reaches 80 °C, gas bubbles begin to evolve; at this moment the heating is stopped. The reaction proceeds with the violent evolution of carbon dioxide. Be careful! If the reaction is too slow, then the temperature of the mixture is gradually brought to 85 °C (when heated above 85 °C, the mixture foams strongly, which leads to the loss of nitromethane). If the reaction is too vigorous, the flask is slightly cooled with a soaked cloth in cold water and the heating is removed. In this case, the decomposition of the sodium salt of nitroacetic acid occurs so quickly that no further heating is required.
At a temperature of 90 °C, the distillation of nitromethane (and water vapor) begins, which is collected in the receiver. When the temperature drops below 95 °C, the flask is gently heated up to 110 °C. The distillation is carried out until the release of nitromethane droplets (oily drops) in the distillate ceases, then the receiver is replaced and another 100 ml of water is distilled off. The crude nitromethane (bottom layer) is separated from the water layer in a separatory funnel; the aqueous layer is extracted with ether (about 100 ml) and the extract is attached to the nitromethane. The same procedure is repeated with the second distillation batch of an aqueous solution of nitromethane, the solutions are combined. The liquid is dried over a little amount of calcium chloride (CaCl2) and filtered through a filter paper. Ether is distilled off in a water bath and nitromethane is distilled under atmospheric pressure or vacuum (preferably), collecting the fraction boiling at a temperature of 98 - 101 °C (at atmospheric pressure). Yield is 20-24 g (33-39%).
If the time consumed is an important consideration, it is well to note that the spontaneous heating from 85 °C to 100 °C gives three-fourths of all the nitromethane obtained in the preparation. This takes less than one hour. The further heating above 100 °C and the distillations of the water layers take over two hours, and give only one-fourth of the total yield.
Fig.1
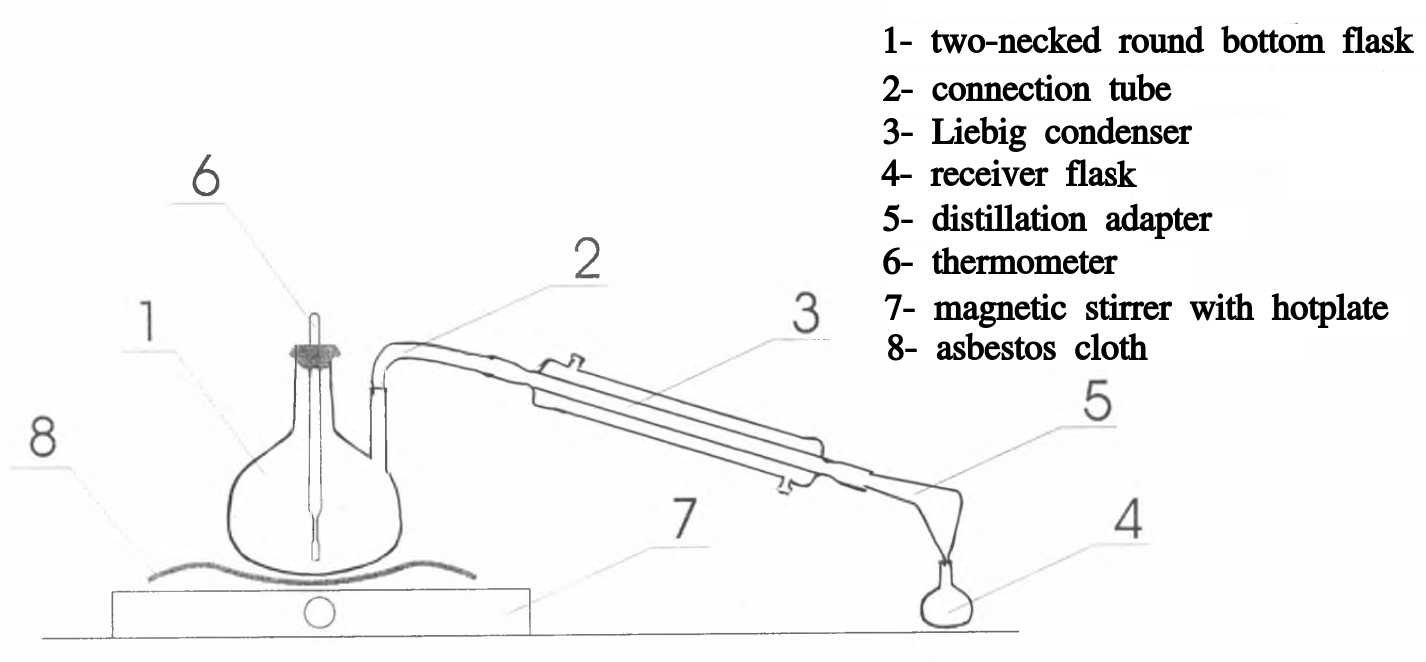
Reagents:
- 97.2 g Monochloroacetic acid;
- 420 ml Distilled water;
- 60 g Anhydrous sodium carbonate (Na2CO3);
- 71.1 g Sodium nitrite;
- 100 g Anhydrous calcium chloride (CaCl2).
Nitromethane synthesis from DMS (dimethyl sulfate)
Into a 2 L two-necked flask equipped with a straight cooler, stirring bar and thermometer place 210 g (2.47 mol) of sodium nitrate, 10 g (0.072 mol) of anhydrous potassium carbonate, 180 ml of distilled water. The mixture is heated at 60 °C with constant stirring. To the resulting solution within 15 minutes, 136 g (1.078 mol) dimethyl sulfate is poured extremely slowly while maintaining the temperature of 58–60 °C with stirring. Cooling is carried out with help of ice water bath. After 1–2 minutes after the end of dimethyl sulfate distilling off, the temperature spontaneously drops. The reaction mixture has to be heating up with oil bath (or heater plate/sand plate).
Distillation begins at a vapor temperature of 30 °C, the main part is distilled at 83–86 °C, then the temperature rises to 110 °C, when water without oil (nitromethane) begins to flow into the receiver. If you want to increase the yield, carry out the second stage. When the temperature in the apparatus reaches 110 °C, salt begins to crystallize on the two-necked flask walls and foam appears; to reduce foaming, at this point 2 g castor oil. At a temperature of 116–117 °C, the second stage of the reaction begins, the liquid in the apparatus looks like a viscous oil, but when the specified temperature is reached, add 100 ml distilled water so that the temperature is in the range of 117-120 °C. Duration of water draining is about 30 minutes. The end of the reaction is recorded by the type of liquid condensing in the refrigerator, as well as by the vapor temperature of 100-100.5 °C. The distillate consists of two layers, the lower saturated solution of water in nitromethane, the upper aqueous solution of nitromethane.
The liquid is dried over a little amount of calcium chloride (CaCl2) and filtered through a filter paper. Ether is distilled off in a water bath and nitromethane is distilled under atmospheric pressure or vacuum (preferably), collecting the fraction boiling at a temperature of 98 - 101 °C (at atmospheric pressure). Yield is 50-57%.
An important fact is that this method can be used to synthesize nitroEthane. You have to replace dimethyl sulfate with diEthyl sulfate.
Distillation begins at a vapor temperature of 30 °C, the main part is distilled at 83–86 °C, then the temperature rises to 110 °C, when water without oil (nitromethane) begins to flow into the receiver. If you want to increase the yield, carry out the second stage. When the temperature in the apparatus reaches 110 °C, salt begins to crystallize on the two-necked flask walls and foam appears; to reduce foaming, at this point 2 g castor oil. At a temperature of 116–117 °C, the second stage of the reaction begins, the liquid in the apparatus looks like a viscous oil, but when the specified temperature is reached, add 100 ml distilled water so that the temperature is in the range of 117-120 °C. Duration of water draining is about 30 minutes. The end of the reaction is recorded by the type of liquid condensing in the refrigerator, as well as by the vapor temperature of 100-100.5 °C. The distillate consists of two layers, the lower saturated solution of water in nitromethane, the upper aqueous solution of nitromethane.
The liquid is dried over a little amount of calcium chloride (CaCl2) and filtered through a filter paper. Ether is distilled off in a water bath and nitromethane is distilled under atmospheric pressure or vacuum (preferably), collecting the fraction boiling at a temperature of 98 - 101 °C (at atmospheric pressure). Yield is 50-57%.
An important fact is that this method can be used to synthesize nitroEthane. You have to replace dimethyl sulfate with diEthyl sulfate.
Reagents:
- 210 g Sodium nitrate;
- 10 g Anhydrous potassium carbonate;
- 350 ml Distilled water;
- 136 g Dimethyl sulfate
- 2 g castor oil (optional);
- 100 g Anhydrous calcium chloride (CaCl2).
Last edited:
