William D.
Expert
- Joined
- Jul 19, 2021
- Messages
- 1,055
- Reaction score
- 1,313
- Points
- 113
2-Amino-1-(4-methylphenyl)propan-1-ol (4-methylnorephedrine).
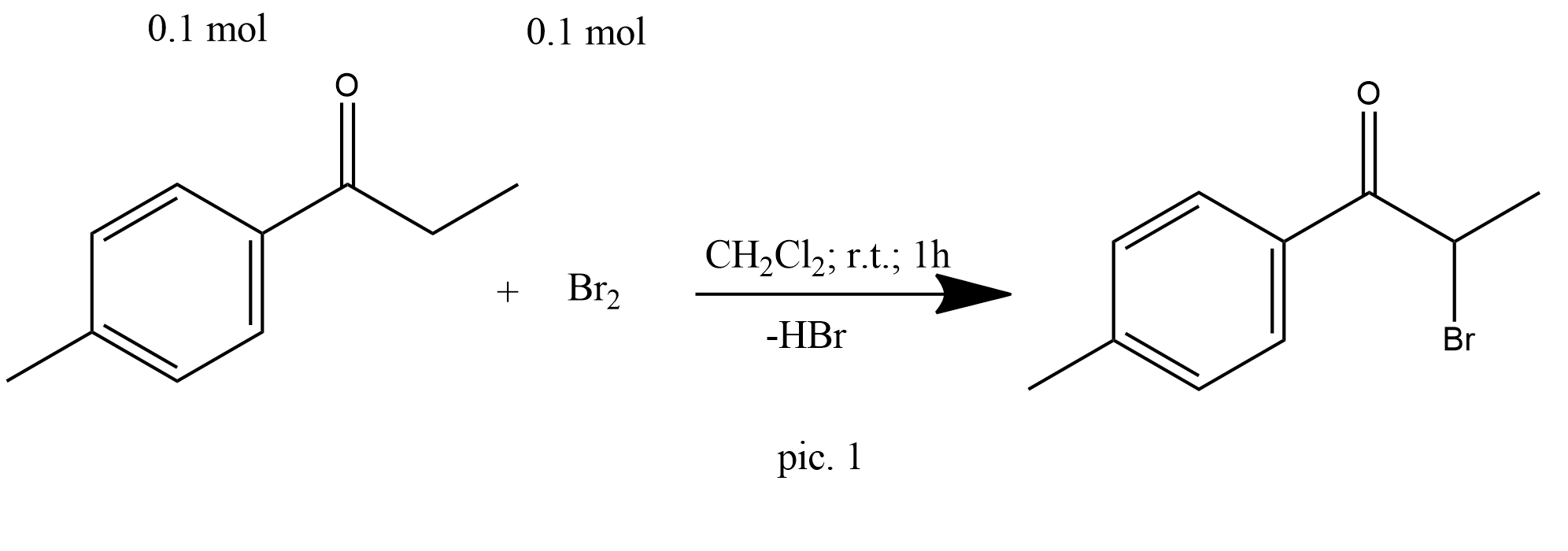
Bromine (5.16 mL, 100 mmol) in dichloromethane (50 mL) was added dropwise to a solution of 4-methylpropiophenone (14.82 g, 100 mmol) in dichloromethane (100 mL). The mixture was then stirred for 1 h at room temperature and dried (anhydrous magnesium sulphate). Following removal of the solvent, α-bromo-4-methylpropiophenone (21.49 g, 94.6 mmol, 95%) remained as yellow crystals (pic.1).
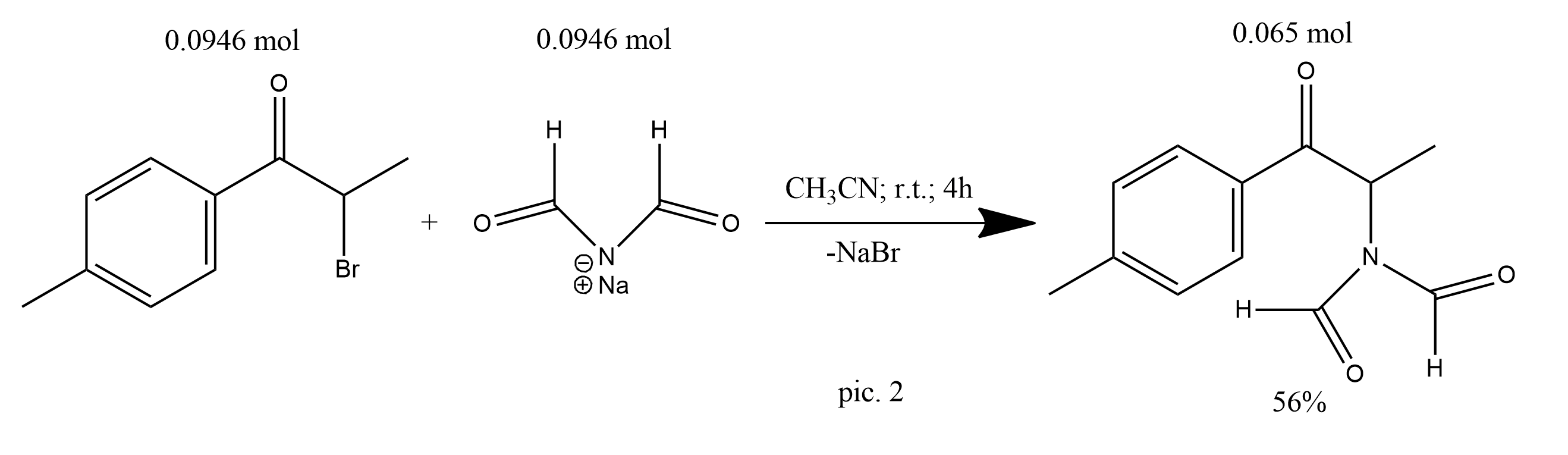
The alpha bromo ketone (20.98 g, 92.4 mmol) was dissolved in acetonitrile (100 mL) and sodium diformylamide (11.01 g, 116 mmol) was added. The mixture was refluxed for 4 hs. Following removal of the solvent, the residue was partitioned between dichloromethane and water. The organic layer was collected, dried (anhydrous magnesium sulphate) and the solvent was removed to give the N, N–diformylamide derivative (14.26 g, 65.0 mmol, 56%) as a yellow oil (pic.2).
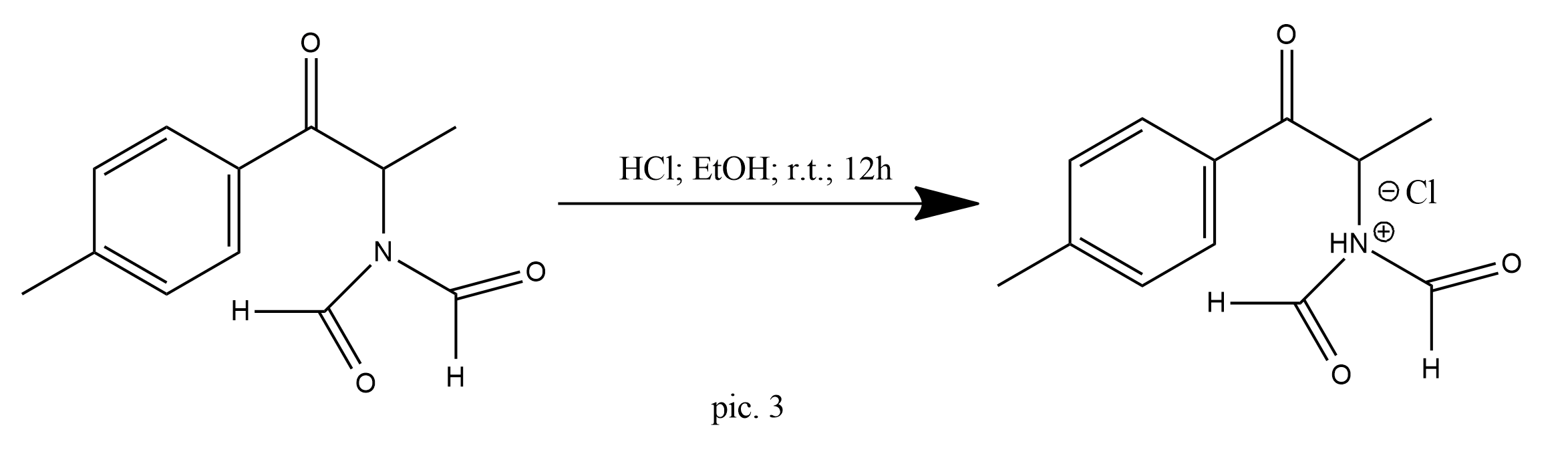
This was dissolved in 5% ethanolic hydrochloric acid (200 mL) and stirred overnight at room temperature (pic.3).
(±)-cis-4-Methyl-5-(4-methylphenyl)-4,5-dihydrooxazol-2-amine ((±)-cis- 4,4′-DMAR).
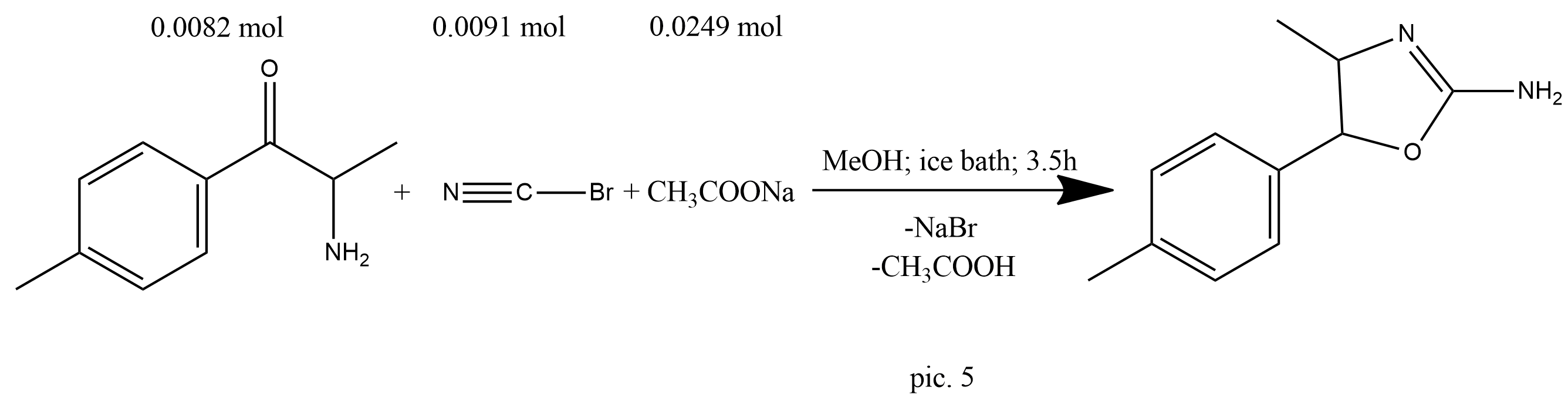
A solution of cyanogen bromide (0.963 g, 9.1 mmol) in methanol (3 mL) was added to a mixture of 2-amino-1-(4-methylphenyl)propan-1-ol (1.36 g, 8.2 mmol) and anhydrous sodium acetate (2.04 g, 24.9 mmol) in methanol (20 mL) with cooling in an ice bath. The mixture was stirred for 3.5 h, the volatiles were removed, and saturated sodium carbonate was added to the residue. The mixture was shaken until a white precipitate formed. This was filtered to afford a colorless solid (1.52 g) (pic.5).
Bromine (5.16 mL, 100 mmol) in dichloromethane (50 mL) was added dropwise to a solution of 4-methylpropiophenone (14.82 g, 100 mmol) in dichloromethane (100 mL). The mixture was then stirred for 1 h at room temperature and dried (anhydrous magnesium sulphate). Following removal of the solvent, α-bromo-4-methylpropiophenone (21.49 g, 94.6 mmol, 95%) remained as yellow crystals (pic.1).
The alpha bromo ketone (20.98 g, 92.4 mmol) was dissolved in acetonitrile (100 mL) and sodium diformylamide (11.01 g, 116 mmol) was added. The mixture was refluxed for 4 hs. Following removal of the solvent, the residue was partitioned between dichloromethane and water. The organic layer was collected, dried (anhydrous magnesium sulphate) and the solvent was removed to give the N, N–diformylamide derivative (14.26 g, 65.0 mmol, 56%) as a yellow oil (pic.2).
This was dissolved in 5% ethanolic hydrochloric acid (200 mL) and stirred overnight at room temperature (pic.3).
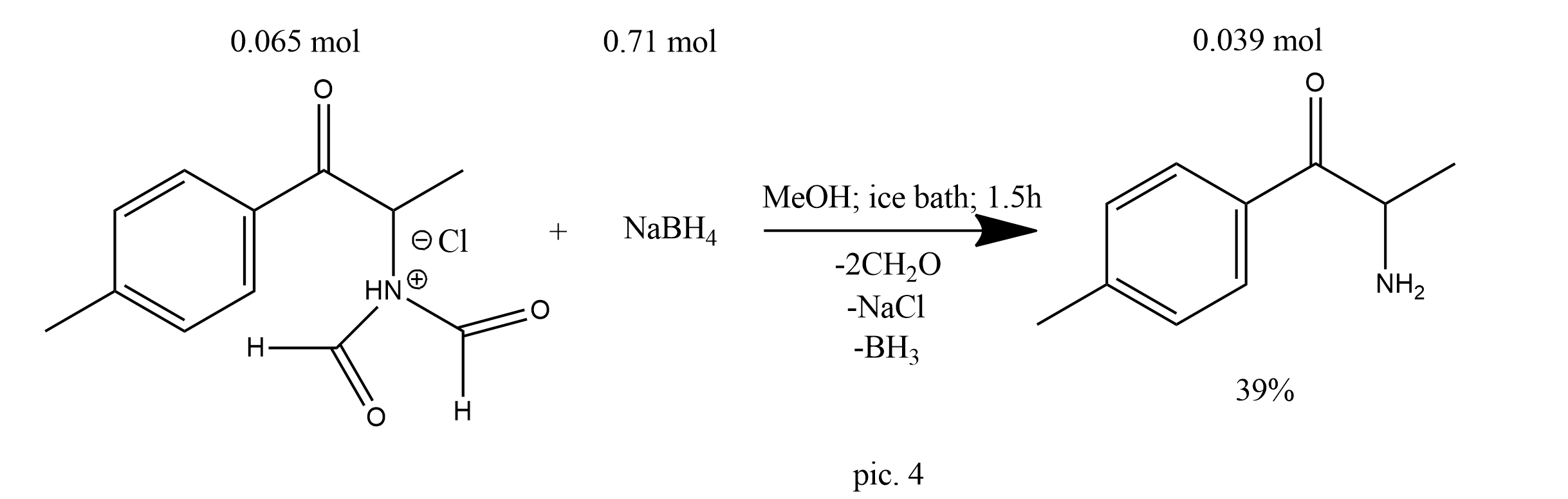
The solution was evaporated to dryness, acetone was added, and the precipitate was collected by filtration. The primary amine intermediate was then used directly without the need for further purification. This was dissolved in methanol (500 mL), cooled in an ice bath and sodium borohydride (26.86 g, 0.71 mol) was added over a 1.5 h period. Removal of the solvent, partitioning between dichloromethane/water, drying (anhydrous magnesium sulphate) and evaporation of the dichloromethane yielded 2-amino-1-(4-methylphenyl)propan-1-ol (6.42 g, 38.9 mmol, 39% from 4-methylpropiophenone) as a colorless solid: m.p. 103-105 °C (pic.4).
(±)-cis-4-Methyl-5-(4-methylphenyl)-4,5-dihydrooxazol-2-amine ((±)-cis- 4,4′-DMAR).
A solution of cyanogen bromide (0.963 g, 9.1 mmol) in methanol (3 mL) was added to a mixture of 2-amino-1-(4-methylphenyl)propan-1-ol (1.36 g, 8.2 mmol) and anhydrous sodium acetate (2.04 g, 24.9 mmol) in methanol (20 mL) with cooling in an ice bath. The mixture was stirred for 3.5 h, the volatiles were removed, and saturated sodium carbonate was added to the residue. The mixture was shaken until a white precipitate formed. This was filtered to afford a colorless solid (1.52 g) (pic.5).
Last edited by a moderator:
