William D.
Expert
- Joined
- Jul 19, 2021
- Messages
- 1,055
- Reaction score
- 1,310
- Points
- 113
Reaction scheme:
Synthesis:
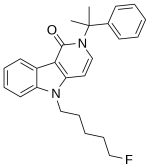
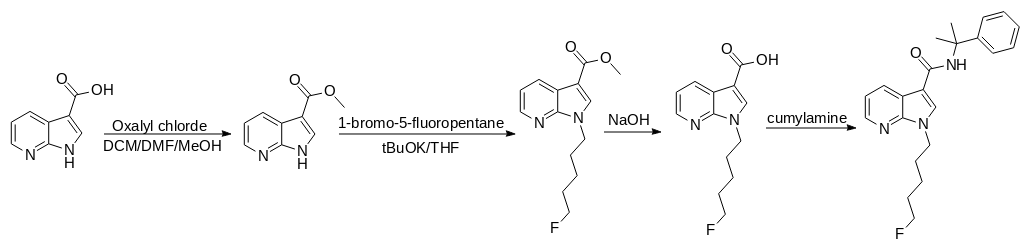
1. A solution of 1H-pyrrolo[2, 3-b]pyridine-3-carboxylic acid (0.49 g, 3 mmol), oxalyl chloride (0.6 mL, 7.2 mmol), and DMF (1 mL) in dichloromethane (20 mL) was stirred for 20 min.
2. The mixture was evaporated and then redissolved in methanol (20 mL) and stirred for 40 min.
3. The mixture was evaporated and then extracted with ethyl acetate.
4. The organic phase was washed with saturated sodium bicarbonate solution and dried with Na2SO4, and the solvent was removed in vacuo to yield the white solid (0.36 g, 68%).
5. A solution of Step 4 (0.36 g, 2 mmol), potassium t-butoxide (0.27 g, 2.4 mmol), and 1-bromo-5-fluoropentane (1.6 g,9.5 mmol) in THF (20 mL) was stirred for 24 h.
6. The mixture was extracted with ethyl acetate.
7. The organic phase was washed with brine and dried with Na2SO4, and the solvent was removed in vacuo.
8. The residue was purified by silica gel chromatography with stepwise gradient elution of n-hexane/ethyl acetate (3:1–2:1–1:1, v/v) to yield the white solid (0.35 g, 65%).
9. A solution of step 8 (0.35 g, 1.3 mmol) and 6 mol/L sodium hydroxide solution (10 mL) in methanol/THF (2:1,v/v, 15 mL) was stirred for 1.5 days.
10. The mixture was evaporated, added to water, and then washed with ethyl acetate.
11. A solution of 1 mol/L hydrochloric acid was added to the aqueous phase, and the mixture was extracted with ethyl acetate.
12. The organic phase was washed with brine and dried with Na2SO4, and the solvent was removed in vacuo to yield the white solid (0.39 g, quant.)
13. A solution of step 12 (0.39 g, 1.3 mmol), oxalyl chloride (3 mmol), and DMF (1 mL) in dichloromethane (12 mL) was stirred for 10 min.
14. The mixture was evaporated and then redissolved in dichloromethane (10 mL).
15. Cumylamine (2 mmol) and triethylamine (0.5 mL) were added to the solution and stirred for 15 h.
16. The mixture was evaporated and then extracted with ethyl acetate.
17. The organic phase was washed with 0.1 mol/L hydrochloric acid solution, saturated sodium bicarbonate solution and brine, and then dried with Na2SO4, and the solvent was removed in vacuo to yield the white solid (0.37 g, 77%).
Synthesis:
1. A solution of 1H-pyrrolo[2, 3-b]pyridine-3-carboxylic acid (0.49 g, 3 mmol), oxalyl chloride (0.6 mL, 7.2 mmol), and DMF (1 mL) in dichloromethane (20 mL) was stirred for 20 min.
2. The mixture was evaporated and then redissolved in methanol (20 mL) and stirred for 40 min.
3. The mixture was evaporated and then extracted with ethyl acetate.
4. The organic phase was washed with saturated sodium bicarbonate solution and dried with Na2SO4, and the solvent was removed in vacuo to yield the white solid (0.36 g, 68%).
5. A solution of Step 4 (0.36 g, 2 mmol), potassium t-butoxide (0.27 g, 2.4 mmol), and 1-bromo-5-fluoropentane (1.6 g,9.5 mmol) in THF (20 mL) was stirred for 24 h.
6. The mixture was extracted with ethyl acetate.
7. The organic phase was washed with brine and dried with Na2SO4, and the solvent was removed in vacuo.
8. The residue was purified by silica gel chromatography with stepwise gradient elution of n-hexane/ethyl acetate (3:1–2:1–1:1, v/v) to yield the white solid (0.35 g, 65%).
9. A solution of step 8 (0.35 g, 1.3 mmol) and 6 mol/L sodium hydroxide solution (10 mL) in methanol/THF (2:1,v/v, 15 mL) was stirred for 1.5 days.
10. The mixture was evaporated, added to water, and then washed with ethyl acetate.
11. A solution of 1 mol/L hydrochloric acid was added to the aqueous phase, and the mixture was extracted with ethyl acetate.
12. The organic phase was washed with brine and dried with Na2SO4, and the solvent was removed in vacuo to yield the white solid (0.39 g, quant.)
13. A solution of step 12 (0.39 g, 1.3 mmol), oxalyl chloride (3 mmol), and DMF (1 mL) in dichloromethane (12 mL) was stirred for 10 min.
14. The mixture was evaporated and then redissolved in dichloromethane (10 mL).
15. Cumylamine (2 mmol) and triethylamine (0.5 mL) were added to the solution and stirred for 15 h.
16. The mixture was evaporated and then extracted with ethyl acetate.
17. The organic phase was washed with 0.1 mol/L hydrochloric acid solution, saturated sodium bicarbonate solution and brine, and then dried with Na2SO4, and the solvent was removed in vacuo to yield the white solid (0.37 g, 77%).
Last edited by a moderator: