William D.
Expert
- Joined
- Jul 19, 2021
- Messages
- 1,059
- Reaction score
- 1,332
- Points
- 113
Reagents:
 Synthesis:
Synthesis:
- Indole (cas 120-72-9) 1000 g;
- Dichloromethane (CH2Cl2) 15 l;
- Tin(IV) chloride (SnCl4) 2000 ml;
- 1-Naphthoyl chloride (cas 879-18-5) 1627 g;
- Nitromethane (cas 75-52-5) 10 l;
- Distilled water 20 l;
- Ethyl acetate (EtOAc) 15 l;
- Sodium or magnesium sulphate (Na2SO4 or MgSO4);
- Batch reactor 50 l with a top stirrer;
- Ice bath;
- Vacuum source;
- Rotovap machine;
- Several buckets;
- Laboratory scale (1-1000 g is suitable);
- Funnel;
- Glass rod and spatula;
- Pyrex dish;
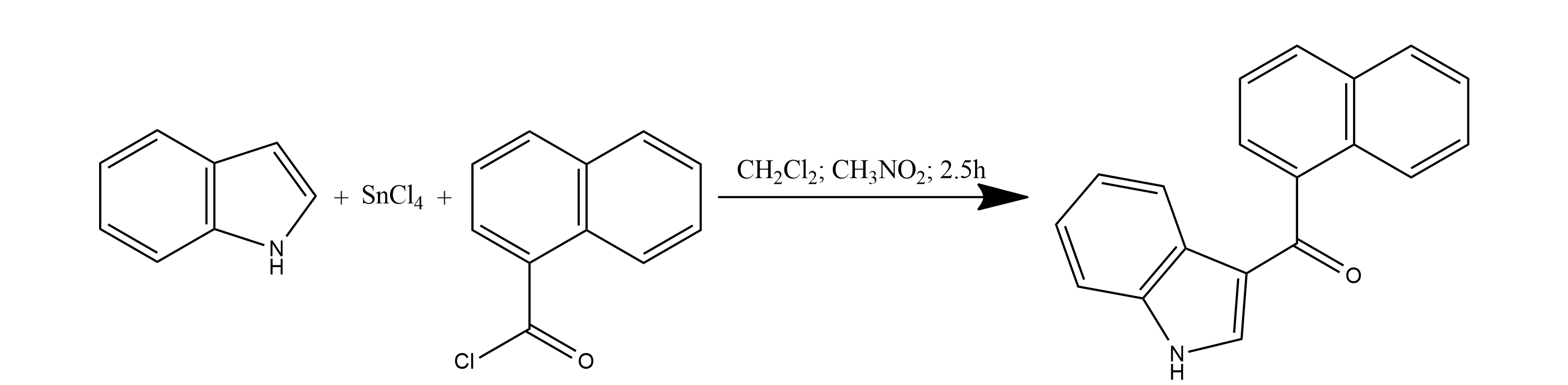
1. Tin(IV) chloride (SnCl4) 2000 ml is added in a single portion to an indole 1000 g solution in dichloromethane (CH2Cl2) 15 l in a batch reactor 50 l.
2. The mixture is stirred at room temperature for 30 min after an ice bath was removed. Then 1-naphthoyl chloride 1627 g is added to the reaction suspension in small portions, followed by nitromethane 10 l.
3. Then, the mixture is stirred for 2 h at room temperature.
4. Next, the reaction mixture is quenched with an ice cold water 20 l. The mixture is filtered in order to remove inorganic precipitates. Layers are separated.
5. The water layer is extracted with ethyl acetate (EtOAc) 15 l. EtOAc extract is combined with organic layer.
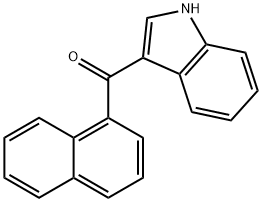
6. The organic phase is dried over sodium or magnesium sulphate (Na2SO4 or MgSO4) and concentrated at reduced pressure. A crystalline solid 3-(1-Naphthoyl)indole product (cas 109555-87-5) is obtained.
2. The mixture is stirred at room temperature for 30 min after an ice bath was removed. Then 1-naphthoyl chloride 1627 g is added to the reaction suspension in small portions, followed by nitromethane 10 l.
3. Then, the mixture is stirred for 2 h at room temperature.
4. Next, the reaction mixture is quenched with an ice cold water 20 l. The mixture is filtered in order to remove inorganic precipitates. Layers are separated.
5. The water layer is extracted with ethyl acetate (EtOAc) 15 l. EtOAc extract is combined with organic layer.
6. The organic phase is dried over sodium or magnesium sulphate (Na2SO4 or MgSO4) and concentrated at reduced pressure. A crystalline solid 3-(1-Naphthoyl)indole product (cas 109555-87-5) is obtained.

3-(1-Naphthoyl)indole | 109555-87-5
Visit ChemicalBook To find more 3-(1-Naphthoyl)indole(109555-87-5) information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes. You can also browse global...
www.chemicalbook.com
Last edited by a moderator:
